Establishment of a subgenomic replicon for bovine viral diarrhea virus in Huh-7 cells and modulation of interferon-regulated factor 3-mediated antiviral response
- PMID: 15708997
- PMCID: PMC548457
- DOI: 10.1128/JVI.79.5.2788-2796.2005
Establishment of a subgenomic replicon for bovine viral diarrhea virus in Huh-7 cells and modulation of interferon-regulated factor 3-mediated antiviral response
Abstract
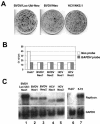
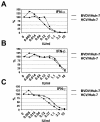
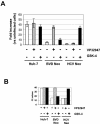
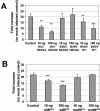
We describe the development of a selectable, bi-cistronic subgenomic replicon for bovine viral diarrhea virus (BVDV) in Huh-7 cells, similar to that established for hepatitis C virus (HCV). The selection marker and reporter (Luc-Ubi-Neo) in the BVDV replicon was fused with the amino-terminal protease N(pro), and expression of the nonstructural proteins (NS3 to NS5B) was driven by an encephalomyocarditis virus internal ribosome entry site. This BVDV replicon allows us to compare RNA replication of these two related viruses in a similar cellular background and to identify antiviral molecules specific for HCV RNA replication. The BVDV replicon showed similar sensitivity as the HCV replicon to interferons (alpha, beta, and gamma) and 2'-beta-C-methyl ribonucleoside inhibitors. Known nonnucleoside inhibitor molecules specific for either HCV or BVDV can be easily distinguished by using the parallel replicon systems. The HCV replicon has been shown to block, via the NS3/4A serine protease, Sendai virus-induced activation of interferon regulatory factor 3 (IRF-3), a key antiviral signaling molecule. Similar suppression of IRF-3-mediated responses was also observed with the Huh-7-BVDV replicon but was independent of NS3/4A protease activity. Instead, the amino-terminal cysteine protease N(pro) of BVDV appears to be, at least partly, responsible for suppressing IRF-3 activation induced by Sendai virus infection. This result suggests that different viruses, including those closely related, may have developed unique mechanisms for evading host antiviral responses. The parallel BVDV and HCV replicon systems provide robust counterscreens to distinguish viral specificity of small-molecule inhibitors of viral replication and to study the interactions of the viral replication machinery with the host cell innate immune system.
Figures



Similar articles
-
Development of a cell-based high-throughput specificity screen using a hepatitis C virus-bovine viral diarrhea virus dual replicon assay.Antimicrob Agents Chemother. 2005 Apr;49(4):1346-53. doi: 10.1128/AAC.49.4.1346-1353.2005. Antimicrob Agents Chemother. 2005. PMID: 15793110 Free PMC article.
-
In vitro antiviral activity of SCH446211 (SCH6), a novel inhibitor of the hepatitis C virus NS3 serine protease.J Antimicrob Chemother. 2007 Jan;59(1):51-8. doi: 10.1093/jac/dkl455. Epub 2006 Dec 5. J Antimicrob Chemother. 2007. PMID: 17151003
-
Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease.Science. 2003 May 16;300(5622):1145-8. doi: 10.1126/science.1082604. Epub 2003 Apr 17. Science. 2003. PMID: 12702807
-
Molecular virology of hepatitis C virus: an update with respect to potential antiviral targets.Antivir Ther. 1998;3(Suppl 3):71-81. Antivir Ther. 1998. PMID: 10726057 Review.
-
Bovine viral diarrhea virus as a surrogate model of hepatitis C virus for the evaluation of antiviral agents.Antiviral Res. 2003 Sep;60(1):1-15. doi: 10.1016/s0166-3542(03)00174-8. Antiviral Res. 2003. PMID: 14516916 Review.
Cited by
-
In Vitro Antiviral Activity and Resistance Profile Characterization of the Hepatitis C Virus NS5A Inhibitor Ledipasvir.Antimicrob Agents Chemother. 2016 Jan 11;60(3):1847-1853. doi: 10.1128/AAC.02524-15. Antimicrob Agents Chemother. 2016. PMID: 26824950 Free PMC article.
-
Ubiquitination and proteasomal degradation of interferon regulatory factor-3 induced by Npro from a cytopathic bovine viral diarrhea virus.Virology. 2007 Sep 30;366(2):277-92. doi: 10.1016/j.virol.2007.04.023. Epub 2007 May 24. Virology. 2007. PMID: 17531282 Free PMC article.
-
An alternative -1/+2 open reading frame exists within viral N(pro)(1-19) region of bovine viral diarrhea virus SD-1.Virus Res. 2012 Jan;163(1):341-51. doi: 10.1016/j.virusres.2011.10.022. Epub 2011 Nov 4. Virus Res. 2012. PMID: 22079882 Free PMC article.
-
The amino-terminal domain of bovine viral diarrhea virus Npro protein is necessary for alpha/beta interferon antagonism.J Virol. 2006 Jan;80(2):900-11. doi: 10.1128/JVI.80.2.900-911.2006. J Virol. 2006. PMID: 16378992 Free PMC article.
-
BVDV Npro protein mediates the BVDV induced immunosuppression through interaction with cellular S100A9 protein.Microb Pathog. 2018 Aug;121:341-349. doi: 10.1016/j.micpath.2018.05.047. Epub 2018 May 31. Microb Pathog. 2018. PMID: 29859294 Free PMC article.
References
-
- Bartenschlager, R. 2002. Hepatitis C virus replicons: potential role for drug development. Nat. Rev. Drug Discov. 1:911-916. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

