Puma lentivirus is controlled in domestic cats after mucosal exposure in the absence of conventional indicators of immunity
- PMID: 15708998
- PMCID: PMC548441
- DOI: 10.1128/JVI.79.5.2797-2806.2005
Puma lentivirus is controlled in domestic cats after mucosal exposure in the absence of conventional indicators of immunity
Abstract
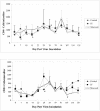
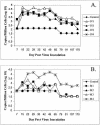
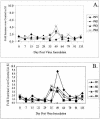
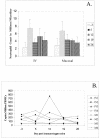
A high percentage of free-ranging pumas (Felis concolor) are infected with feline lentiviruses (puma lentivirus, feline immunodeficiency virus Pco [FIV-Pco], referred to here as PLV) without evidence of disease. PLV establishes productive infection in domestic cats following parenteral exposure but, in contrast to domestic cat FIV, it does not cause T-cell dysregulation. Here we report that cats exposed to PLV oro-nasally became infected yet rapidly cleared peripheral blood mononuclear cell (PBMC) proviral load in the absence of a correlative specific immune response. Two groups of four specific-pathogen-free cats were exposed to PLV via the mucosal (oro-nasal) or parenteral (i.v.) route. All animals were PBMC culture positive and PCR positive within 3 weeks postinfection and seroconverted without exhibiting clinical disease; however, three or four oro-nasally infected animals cleared circulating proviral DNA within 3 months. Antibody titers reached higher levels in animals that remained persistently infected. PLV antigen-induced proliferation was slightly greater in mucosally inoculated animals, but no differences were noted in cytotoxic T-lymphocyte responses or cytokine profiles between groups. The distribution of virus was predominantly gastrointestinal as opposed to lymphoid in all animals in which virus was detected at necropsy. Possible mechanisms for viral clearance include differences in viral fitness required for crossing mucosal surfaces, a threshold dose requirement for persistence, or an undetected sterilizing host immune response. This is the first report of control of a productive feline or primate lentivirus infection in postnatally exposed, seropositive animals. Mechanisms underlying this observation will provide clues to containment of immunodeficiency disease and could prompt reexamination of vaccine-induced immunity against human immunodeficiency virus and other lentiviruses.
Figures


Similar articles
-
Domestic cats infected with lion or puma lentivirus develop anti-feline immunodeficiency virus immune responses.J Acquir Immune Defic Syndr. 2003 Sep 1;34(1):20-31. doi: 10.1097/00126334-200309010-00003. J Acquir Immune Defic Syndr. 2003. PMID: 14501789
-
Prior Puma Lentivirus Infection Modifies Early Immune Responses and Attenuates Feline Immunodeficiency Virus Infection in Cats.Viruses. 2018 Apr 20;10(4):210. doi: 10.3390/v10040210. Viruses. 2018. PMID: 29677149 Free PMC article.
-
Prior virus exposure alters the long-term landscape of viral replication during feline lentiviral infection.Viruses. 2011 Oct;3(10):1891-908. doi: 10.3390/v3101891. Epub 2011 Oct 13. Viruses. 2011. PMID: 22069521 Free PMC article.
-
Mucosal infection and vaccination against feline immunodeficiency virus.J Biotechnol. 1999 Aug 20;73(2-3):213-21. doi: 10.1016/s0168-1656(99)00139-x. J Biotechnol. 1999. PMID: 10486930 Review.
-
Restrictions to cross-species transmission of lentiviral infection gleaned from studies of FIV.Vet Immunol Immunopathol. 2010 Mar 15;134(1-2):25-32. doi: 10.1016/j.vetimm.2009.10.005. Epub 2009 Oct 14. Vet Immunol Immunopathol. 2010. PMID: 19896218 Free PMC article. Review.
Cited by
-
Variation in Intra-individual Lentiviral Evolution Rates: a Systematic Review of Human, Nonhuman Primate, and Felid Species.J Virol. 2019 Jul 30;93(16):e00538-19. doi: 10.1128/JVI.00538-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31167917 Free PMC article.
-
Species-specific differences in the ability of feline lentiviral Vif to degrade feline APOBEC3 proteins.Microbiol Immunol. 2016 Apr;60(4):272-9. doi: 10.1111/1348-0421.12371. Microbiol Immunol. 2016. PMID: 26935128 Free PMC article.
-
Error correction and statistical analyses for intra-host comparisons of feline immunodeficiency virus diversity from high-throughput sequencing data.BMC Bioinformatics. 2015 Jun 30;16:202. doi: 10.1186/s12859-015-0607-z. BMC Bioinformatics. 2015. PMID: 26123018 Free PMC article.
-
Going wild: lessons from naturally occurring T-lymphotropic lentiviruses.Clin Microbiol Rev. 2006 Oct;19(4):728-62. doi: 10.1128/CMR.00009-06. Clin Microbiol Rev. 2006. PMID: 17041142 Free PMC article. Review.
-
Prevention of immunodeficiency virus induced CD4+ T-cell depletion by prior infection with a non-pathogenic virus.Virology. 2008 Jul 20;377(1):63-70. doi: 10.1016/j.virol.2008.03.037. Epub 2008 May 22. Virology. 2008. PMID: 18499211 Free PMC article.
References
-
- Abel, K., L. La Franco-Scheuch, T. Rourke, Z.-M. Ma, V. De Silva, B. Fallert, L. Beckett, T. A. Reinhart, and C. J. Miller. 2004. Gamma interferon-mediated inflammation is associated with lack of protection from intravaginal simian immunodeficiency virus SIVmac239 challenge in simian-human immunodeficiency virus 89.6-immunized rhesus macaques. J. Virol. 78:841-854. - PMC - PubMed
-
- Barr, M. C., P. P. Calle, M. E. Roelke, and F. W. Scott. 1989. Feline immunodeficiency virus infection in nondomestic felids. J. Zoo Wildl. Med. 20:265-272.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

