Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection
- PMID: 15709001
- PMCID: PMC548453
- DOI: 10.1128/JVI.79.5.2823-2830.2005
Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection
Abstract
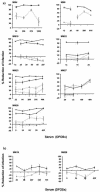
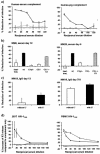
Specific CD8 T-cell responses to human immunodeficiency virus type 1 (HIV-1) are induced in primary infection and make an important contribution to the control of early viral replication. The importance of neutralizing antibodies in containing primary viremia is questioned because they usually arise much later. Nevertheless antienvelope antibodies develop simultaneously with, or even before, peak viremia. We determined whether such antibodies might control viremia by complement-mediated inactivation (CMI). In each of seven patients studied, antibodies capable of CMI appeared at or shortly after the peak in viremia, concomitantly with detection of virus-specific T-cell responses. The CMI was effective on both autologous and heterologous HIV-1 isolates. Activation of the classical pathway and direct viral lysis were at least partly responsible. Since immunoglobulin G (IgG)-antibodies triggered the CMI, specific memory B cells could also be induced by vaccination. Thus, consideration should be given to vaccination strategies that induce IgG antibodies capable of CMI.
Figures

References
-
- Aasa-Chapman, M. M. I., A. Hayman, P. Newton, D. Cornforth, I. Williams, P. Borrow, P. Balfe, and A. McKnight. 2004. Development of the antibody response in acute HIV-1 infection. AIDS 18:371-381. - PubMed
-
- Albert, J., B. Abrahamsson, K. Nagy, E. Aurelius, H. Gaines, G. Nystrom, and E. M. Fenyo. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4:107-112. - PubMed
-
- Bajtay, Z., C. Speth, A. Erdei, and M. P. Dierich. 2004. Cutting edge: productive HIV-1 infection of dendritic cells via complement receptor type 3 (CR3, CD11b/CD18). J. Immunol. 173:4775-4778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

