Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus
- PMID: 15709004
- PMCID: PMC548444
- DOI: 10.1128/JVI.79.5.2847-2858.2005
Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus
Abstract
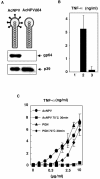
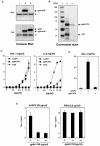
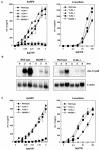
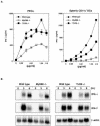
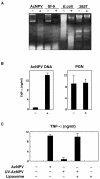
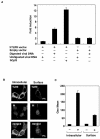
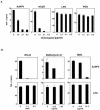
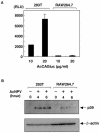
We have previously shown that mice inoculated intranasally with a wild-type baculovirus (Autographa californica nuclear polyhedrosis virus [AcNPV]) are protected from a lethal challenge by influenza virus. However, the precise mechanism of induction of this protective immune response by the AcNPV treatment remained unclear. Here we show that AcNPV activates immune cells via the Toll-like receptor 9 (TLR9)/MyD88-dependent signaling pathway. The production of inflammatory cytokines was severely reduced in peritoneal macrophages (PECs) and splenic CD11c(+) dendritic cells (DCs) derived from mice deficient in MyD88 or TLR9 after cultivation with AcNPV. In contrast, a significant amount of alpha interferon (IFN-alpha) was still detectable in the PECs and DCs of these mice after stimulation with AcNPV, suggesting that a TLR9/MyD88-independent signaling pathway might also participate in the production of IFN-alpha by AcNPV. Since previous work showed that TLR9 ligands include bacterial DNA and certain oligonucleotides containing unmethylated CpG dinucleotides, we also examined the effect of baculoviral DNA on the induction of innate immunity. Transfection of the murine macrophage cell line RAW264.7 with baculoviral DNA resulted in the production of the inflammatory cytokine, while the removal of envelope glycoproteins from viral particles, UV irradiation of the virus, and pretreatment with purified baculovirus envelope proteins or endosomal maturation inhibitors diminished the induction of the immune response by AcNPV. Together, these results indicate that the internalization of viral DNA via membrane fusion mediated by the viral envelope glycoprotein, as well as endosomal maturation, which releases the viral genome into TLR9-expressing cellular compartments, is necessary for the induction of the innate immune response by AcNPV.
Figures
Similar articles
-
The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets.J Immunol. 2003 Mar 15;170(6):3059-64. doi: 10.4049/jimmunol.170.6.3059. J Immunol. 2003. PMID: 12626561
-
Intracellular bacterial infection-induced IFN-gamma is critically but not solely dependent on Toll-like receptor 4-myeloid differentiation factor 88-IFN-alpha beta-STAT1 signaling.J Immunol. 2004 May 15;172(10):6345-53. doi: 10.4049/jimmunol.172.10.6345. J Immunol. 2004. PMID: 15128825
-
Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway.J Immunol. 2004 Apr 15;172(8):4926-33. doi: 10.4049/jimmunol.172.8.4926. J Immunol. 2004. PMID: 15067072
-
Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice.Trends Immunol. 2001 Feb;22(2):78-83. doi: 10.1016/s1471-4906(00)01811-1. Trends Immunol. 2001. PMID: 11286707 Review.
-
The immunobiology of the TLR9 subfamily.Trends Immunol. 2004 Jul;25(7):381-6. doi: 10.1016/j.it.2004.04.011. Trends Immunol. 2004. PMID: 15207506 Review. No abstract available.
Cited by
-
Head-to-head comparison of three vaccination strategies based on DNA and raw insect-derived recombinant proteins against Leishmania.PLoS One. 2012;7(12):e51181. doi: 10.1371/journal.pone.0051181. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236448 Free PMC article.
-
Rotavirus Recombinant VP6 Nanotubes Act as an Immunomodulator and Delivery Vehicle for Norovirus Virus-Like Particles.J Immunol Res. 2016;2016:9171632. doi: 10.1155/2016/9171632. Epub 2016 Sep 4. J Immunol Res. 2016. PMID: 27689099 Free PMC article.
-
Pulmonary mucosal immunity mediated through CpG provides adequate protection against pulmonary Mycobacterium tuberculosis infection in the mouse model. A role for type I interferon.Tuberculosis (Edinb). 2020 Jul;123:101949. doi: 10.1016/j.tube.2020.101949. Epub 2020 Jun 6. Tuberculosis (Edinb). 2020. PMID: 32741537 Free PMC article.
-
Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation.Blood. 2008 Nov 1;112(9):3713-22. doi: 10.1182/blood-2008-03-146290. Epub 2008 Aug 12. Blood. 2008. PMID: 18698004 Free PMC article.
-
Mucosal delivery of ACNPV baculovirus driving expression of the Gal-lectin LC3 fragment confers protection against amoebic liver abscess in hamster.Int J Biol Sci. 2011;7(9):1345-56. doi: 10.7150/ijbs.7.1345. Epub 2011 Nov 1. Int J Biol Sci. 2011. PMID: 22110386 Free PMC article.
References
-
- Abe, T., H. Takahashi, H. Hamazaki, N. Miyano-Kurosaki, Y. Matsuura, and H. Takaku. 2003. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 171:1133-1139. - PubMed
-
- Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. - PubMed
-
- Ahmad-Nejad, P., H. Hacker, M. Rutz, S. Bauer, R. M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptor at distinct cellular compartments. Eur. J. Immunol. 32:1958-1968. - PubMed
-
- Airenne, K. J., M. O. Hiltunen, M. P. Turunen, A. M. Turunen, O. H. Laitinen, M. S. Kulomaa, and S. Y. Herttuala. 2000. Baculovirus-mediated periadventitial gene transfer to rabbit carotid artery. Gene Ther. 7:1499-1504. - PubMed
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

