The ectodomain of herpes simplex virus glycoprotein H contains a membrane alpha-helix with attributes of an internal fusion peptide, positionally conserved in the herpesviridae family
- PMID: 15709012
- PMCID: PMC548475
- DOI: 10.1128/JVI.79.5.2931-2940.2005
The ectodomain of herpes simplex virus glycoprotein H contains a membrane alpha-helix with attributes of an internal fusion peptide, positionally conserved in the herpesviridae family
Erratum in
- J Virol. 2007 Mar;81(5):2539
Abstract
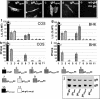
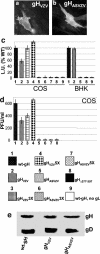
Human herpesviruses enter cells by fusion with target membranes, a process that requires three conserved glycoproteins: gB, gH, and gL. How these glycoproteins execute fusion is unknown. Neural network bioinformatics predicted a membrane alpha-helix contained within the ectodomain of herpes simplex virus (HSV) gH, positionally conserved in the gH of all examined herpesviruses. Evidence that it has attributes of an internal fusion peptide rests on the following lines of evidence. (i) The predicted membrane alpha-helix has the attribute of a membrane segment, since it transformed a soluble form of gD into a membrane-bound gD. (ii) It represents a critical domain of gH. Its partial or entire deletion, or substitution of critical residues inhibited HSV infectivity and fusion in the cell-cell fusion assay. (iii) Its replacement with the fusion peptide from human immunodeficiency virus gp41 or from vesicular stomatitis virus G partially rescued HSV infectivity and cell-cell fusion. The corresponding antisense sequences did not. (iv) The predicted alpha-helix located in the varicella-zoster virus gH ectodomain can functionally substitute the native HSV gH membrane alpha-helix, suggesting a conserved function in the human herpesviruses. We conclude that HSV gH exhibits features typical of viral fusion glycoproteins and that this property is likely conserved in the Herpesviridae family.
Figures


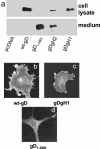

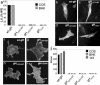
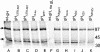
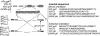
References
-
- Avitabile, E., G. Lombardi, T. Gianni, M. Capri, and G. Campadelli-Fiume. 2004. Coexpression of UL20p and gK inhibits cell-cell fusion mediated by herpes simplex virus glycoproteins gD, gH-gL, and wt-gB or an endocytosis-defective gB mutant, and downmodulates their cell surface expression. J. Virol. 78:8015-8025. - PMC - PubMed
-
- Bzik, D. J., B. A. Fox, N. A. DeLuca, and S. Person. 1984. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology 137:185-190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

