Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys
- PMID: 15709015
- PMCID: PMC548456
- DOI: 10.1128/JVI.79.5.2956-2963.2005
Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys
Abstract
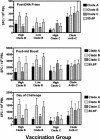
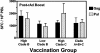
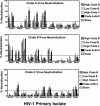
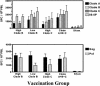
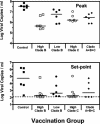
The development of a human immunodeficiency virus type 1 (HIV-1) vaccine that elicits potent cellular and humoral immune responses recognizing divergent strains of HIV-1 will be critical for combating the global AIDS epidemic. The present studies were initiated to examine the magnitude and breadth of envelope (Env)-specific T-lymphocyte and antibody responses generated by vaccines containing either a single or multiple genetically distant HIV-1 Env immunogens. Rhesus monkeys were immunized with DNA prime-recombinant adenovirus boost vaccines encoding a Gag-Pol-Nef polyprotein in combination with either a single Env or a mixture of clade-A, clade-B, and clade-C Envs. Monkeys receiving the multiclade Env immunization developed robust immune responses to all vaccine antigens and, importantly, a greater breadth of Env recognition than monkeys immunized with vaccines including a single Env immunogen. All groups of vaccinated monkeys demonstrated equivalent immune protection following challenge with the pathogenic simian-human immunodeficiency virus 89.6P. These data suggest that a multicomponent vaccine encoding Env proteins from multiple clades of HIV-1 can generate broad Env-specific T-lymphocyte and antibody responses without antigenic interference. This study demonstrates that it is possible to generate protective immune responses by vaccination with genetically diverse isolates of HIV-1.
Figures


References
-
- Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. - PubMed
-
- Chakrabarti, B. K., W. P. Kong, B. Y. Wu, Z. Y. Yang, J. R. Mascola, and G. J. Nabel. Expanded breadth of virus neutralization after immunization with a multiclade envelope HIV vaccine candidate. Submitted for publication. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

