DNA damage sensors ATM, ATR, DNA-PKcs, and PARP-1 are dispensable for human immunodeficiency virus type 1 integration
- PMID: 15709017
- PMCID: PMC548471
- DOI: 10.1128/JVI.79.5.2973-2978.2005
DNA damage sensors ATM, ATR, DNA-PKcs, and PARP-1 are dispensable for human immunodeficiency virus type 1 integration
Abstract
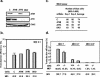
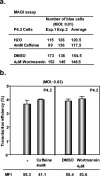
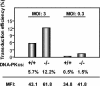
Integration of a DNA copy of the viral RNA genome is a crucial step in the life cycle of human immunodeficiency virus type 1 (HIV-1) and other retroviruses. While the virally encoded integrase is key to this process, cellular factors yet to be characterized are suspected to participate in its completion. DNA damage sensors such as ATM (ataxia-telangiectasia mutated), ATR (ATM- and Rad3-related), DNA-PK (DNA-dependent protein kinase), and PARP-1 [poly(ADP-ribose) polymerase 1] play central roles in responses to various forms of DNA injury and as such could facilitate HIV integration. To test this hypothesis, we examined the susceptibility to infection with wild-type HIV-1 and to transduction with a vesicular stomatitis virus G protein (VSV-G)-pseudotyped HIV-1-derived lentiviral vector of human cells stably expressing small interfering RNAs against ATM, ATR, and PARP-1. We found that integration normally occurred in these knockdown cells. Similarly, the VSV-G-pseudotyped HIV-1-based vector could effectively transduce ATM and PARP-1 knockout mouse cells as well as human cells deficient for DNA-PK. Finally, treatment of target cells with the ATM and ATR inhibitors caffeine and wortmannin was without effect in these infectivity assays. We conclude that the DNA repair enzymes ATM, ATR, DNA-PKcs, and PARP-1 are not essential for HIV-1 integration.
Figures


References
-
- Ariumi, Y., M. Masutani, T. D. Copeland, T. Mimori, T. Sugimura, K. Shimotohno, K. Ueda, M. Hatanaka, and M. Noda. 1999. Suppression of the poly(ADP-ribose) polymerase activity by DNA-dependent protein kinase in vitro. Oncogene 18:4616-4625. - PubMed
-
- Bridge, A. J., S. Pebernard, A. Ducraux, A.-L. Nicoulaz, and R. Iggo. 2003. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 34:263-264. - PubMed
-
- Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. - PubMed
-
- Charneau, P., G. Mirambeau, P. Roux, S. Paulous, H. Buc, and F. Clavel. 1994. HIV-1 reverse transcription. A termination step at the center of the genome. J. Mol. Biol. 241:651-662. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

