Immune tolerance split between hepatitis B virus precore and core proteins
- PMID: 15709022
- PMCID: PMC548461
- DOI: 10.1128/JVI.79.5.3016-3027.2005
Immune tolerance split between hepatitis B virus precore and core proteins
Abstract
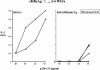
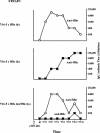
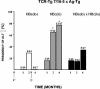
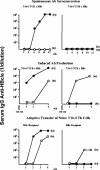
The function of the hepatitis B virus (HBV) precore or HBeAg is largely unknown because it is not required for viral assembly, infection, or replication. However, the HBeAg does appear to play a role in viral persistence. It has been suggested that the HBeAg may promote HBV chronicity by functioning as an immunoregulatory protein. As a model of chronic HBeAg exposure and to examine the tolerogenic potential of the HBV precore and core (HBcAg) proteins, HBc/HBeAg-transgenic (Tg) mice crossed with T cell receptor (TCR)-Tg mice expressing receptors for the HBc/HBeAgs (i.e., TCR-antigen double-Tg pairs) were produced. This study revealed three phenotypes of HBe/HBcAg-specific T-cell tolerance: (i) profound T-cell tolerance most likely mediated by clonal deletion, (ii) T-cell clonal ignorance, and (iii) nondeletional T-cell tolerance mediated by clonal anergy and dependent on the structure, location, and concentration of the tolerogen. The secreted HBeAg is significantly more efficient than the intracellular HBcAg at eliciting T-cell tolerance. The split T-cell tolerance between the HBeAg and the HBcAg and the clonal heterogeneity of HBc/HBeAg-specific T-cell tolerance may have significant implications for natural HBV infection and especially for precore-negative chronic hepatitis.
Figures

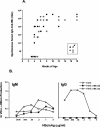
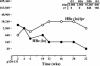
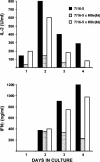
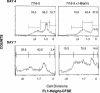

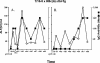
Similar articles
-
A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen.Proc Natl Acad Sci U S A. 2004 Oct 12;101(41):14913-8. doi: 10.1073/pnas.0406282101. Epub 2004 Oct 5. Proc Natl Acad Sci U S A. 2004. PMID: 15469922 Free PMC article.
-
The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: a mechanism for persistence.J Immunol. 1998 Feb 15;160(4):2013-21. J Immunol. 1998. PMID: 9469465
-
Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero?Proc Natl Acad Sci U S A. 1990 Sep;87(17):6599-603. doi: 10.1073/pnas.87.17.6599. Proc Natl Acad Sci U S A. 1990. PMID: 2395863 Free PMC article.
-
Role of T-cell tolerance in the persistence of hepatitis B virus infection.J Immunother Emphasis Tumor Immunol. 1993 Oct;14(3):226-33. doi: 10.1097/00002371-199310000-00010. J Immunother Emphasis Tumor Immunol. 1993. PMID: 8297904 Review.
-
Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection.Hepatology. 2003 Nov;38(5):1075-86. doi: 10.1053/jhep.2003.50453. Hepatology. 2003. PMID: 14578844 Review.
Cited by
-
Efficacy of lamivudine or entecavir against virological rebound after achieving HBV DNA negativity in chronic hepatitis B patients.Int J Med Sci. 2013;10(6):647-52. doi: 10.7150/ijms.5904. Epub 2013 Apr 1. Int J Med Sci. 2013. PMID: 23569428 Free PMC article.
-
Molecular pathogenesis: Connections between viral hepatitis-induced and non-alcoholic steatohepatitis-induced hepatocellular carcinoma.Front Immunol. 2022 Sep 15;13:984728. doi: 10.3389/fimmu.2022.984728. eCollection 2022. Front Immunol. 2022. PMID: 36189208 Free PMC article. Review.
-
Rational design of a multiepitope vaccine encoding T-lymphocyte epitopes for treatment of chronic hepatitis B virus infections.J Virol. 2008 Jan;82(1):435-50. doi: 10.1128/JVI.01505-07. Epub 2007 Oct 17. J Virol. 2008. PMID: 17942551 Free PMC article.
-
Immune-Escape Hepatitis B Virus Mutations Associated with Viral Reactivation upon Immunosuppression.Viruses. 2019 Aug 24;11(9):778. doi: 10.3390/v11090778. Viruses. 2019. PMID: 31450544 Free PMC article. Review.
-
Biogenesis of hepatitis B virus e antigen is driven by translocon-associated protein complex and regulated by conserved cysteine residues within its signal peptide sequence.FEBS J. 2022 May;289(10):2895-2914. doi: 10.1111/febs.16304. Epub 2021 Dec 18. FEBS J. 2022. PMID: 34839586 Free PMC article.
References
-
- Arakawa, K., F. Tsuda, K. Takahashi, I. Ise, S. Naito, E. Kosugi, Y. Miyakawa, and M. Mayumi. 1982. Maternofetal transmission of IgG-bound hepatitis B e antigen. Pediatr. Res. 16:247-250. - PubMed
-
- Carman, W. F., M. R. Jacyna, S. Hadziyannis, P. Karayiannis, M. J. McGarvey, A. Makris, and H. C. Thomas. 1989. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet ii:588-590. - PubMed
-
- Carman, W. F., E. A. Fagan, S. Hadziyannis, P. Karayiannis, N. C. Tassopoulos, R. Williams, and H. C. Thomas. 1991. Association of a precore genomic variant of hepatitis B virus with fulminant hepatitis. Hepatology 14:219-222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

