Processing and presentation of exogenous HLA class I peptides by dendritic cells from human immunodeficiency virus type 1-infected persons
- PMID: 15709025
- PMCID: PMC548465
- DOI: 10.1128/JVI.79.5.3052-3062.2005
Processing and presentation of exogenous HLA class I peptides by dendritic cells from human immunodeficiency virus type 1-infected persons
Abstract
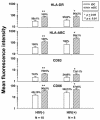
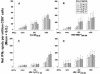
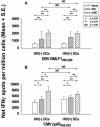
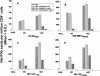
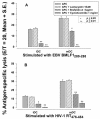
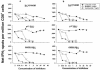
Dendritic cells (DCs) loaded with viral peptides are a potential form of immunotherapy of human immunodeficiency virus type 1 (HIV-1) infection. We show that DCs derived from blood monocytes of subjects with chronic HIV-1 infection on combination antiretroviral drug therapy have increases in expression of HLA, T-cell coreceptor, and T-cell activation molecules in response to the DC maturation factor CD40L comparable to those from uninfected persons. Mature DCs (mDCs) loaded with HLA A*0201-restricted viral peptides of the optimal length (9-mer) were more efficient at activating antiviral CD8(+) T cells than were immature DCs or peptide alone. Optimal presentation of these exogenous peptides required uptake and vesicular trafficking and was comparable in DCs derived from HIV-1-infected and uninfected persons. Furthermore, DCs from HIV-1-infected and uninfected persons had similar capacities to process viral peptides with C-terminal and N-terminal extensions through their proteasomal and cytosolic pathways, respectively. We conclude that DCs derived from HIV-1-infected persons have similar abilities to process exogenous peptides for presentation to CD8(+) T cells as those from uninfected persons. This conclusion supports the use of DCs loaded with synthetic peptides in immunotherapy of HIV-1 infection.
Figures

Similar articles
-
Multiple T-cell responses to human immunodeficiency virus type 1 are enhanced by dendritic cells.Clin Vaccine Immunol. 2009 Oct;16(10):1504-16. doi: 10.1128/CVI.00104-09. Epub 2009 Aug 19. Clin Vaccine Immunol. 2009. PMID: 19692626 Free PMC article.
-
Dendritic cell function during chronic hepatitis C virus and human immunodeficiency virus type 1 infection.Clin Vaccine Immunol. 2007 Sep;14(9):1127-37. doi: 10.1128/CVI.00141-07. Epub 2007 Jul 18. Clin Vaccine Immunol. 2007. PMID: 17634507 Free PMC article.
-
Optimizing the exogenous antigen loading of monocyte-derived dendritic cells.Int Immunol. 2005 May;17(5):621-35. doi: 10.1093/intimm/dxh243. Epub 2005 Apr 11. Int Immunol. 2005. Retraction in: Int Immunol. 2012 Jun;24(6):401. doi: 10.1093/intimm/dxs063. PMID: 15824067 Retracted.
-
The interaction of immunodeficiency viruses with dendritic cells.Curr Top Microbiol Immunol. 2003;276:1-30. doi: 10.1007/978-3-662-06508-2_1. Curr Top Microbiol Immunol. 2003. PMID: 12797441 Review.
-
"HIV-peplotion vaccine"--a novel approach to protection against AIDS by transepithelial transport of viral peptides to Langerhans cells for long-term antiviral CTL response. (A review).Acta Microbiol Immunol Hung. 1996;43(1):1-17. Acta Microbiol Immunol Hung. 1996. PMID: 8806938 Review.
Cited by
-
MHC class I trafficking signal improves induction of cytotoxic T lymphocyte using artificial antigen presenting cells.Biochem Biophys Rep. 2025 Feb 19;41:101946. doi: 10.1016/j.bbrep.2025.101946. eCollection 2025 Mar. Biochem Biophys Rep. 2025. PMID: 40046256 Free PMC article.
-
Multiparametric analyses of human PBMCs loaded ex vivo with a candidate idiotype vaccine for HCV-related lymphoproliferative disorders.PLoS One. 2012;7(9):e44870. doi: 10.1371/journal.pone.0044870. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028651 Free PMC article.
-
Failure of HIV-exposed CD4+ T cells to activate dendritic cells is reversed by restoration of CD40/CD154 interactions.Blood. 2006 Mar 1;107(5):1989-95. doi: 10.1182/blood-2005-07-2731. Epub 2005 Nov 3. Blood. 2006. PMID: 16269614 Free PMC article.
-
Using Dendritic Cell-Based Immunotherapy to Treat HIV: How Can This Strategy be Improved?Front Immunol. 2018 Dec 18;9:2993. doi: 10.3389/fimmu.2018.02993. eCollection 2018. Front Immunol. 2018. PMID: 30619346 Free PMC article. Review.
-
Multiple T-cell responses to human immunodeficiency virus type 1 are enhanced by dendritic cells.Clin Vaccine Immunol. 2009 Oct;16(10):1504-16. doi: 10.1128/CVI.00104-09. Epub 2009 Aug 19. Clin Vaccine Immunol. 2009. PMID: 19692626 Free PMC article.
References
-
- Andrieu, M., E. Loing, J. F. Desoutter, F. Connan, J. Choppin, H. Gras-Masse, D. Hanau, A. Dautry-Varsat, J. G. Guillet, and A. Hosmalin. 2000. Endocytosis of an HIV-derived lipopeptide into human dendritic cells followed by class I-restricted CD8+ T lymphocyte activation. Eur. J. Immunol. 30:3256-3265. - PubMed
-
- Apostolopoulos, V., I. F. C. McKenzie, and I. A. Wilson. 2001. Getting into the groove: unusual features of peptide binding to MHC class I molecules and implications in vaccine design. Front. Biosci. 6:D1311-D1320. - PubMed
-
- Banchereau, J., B. Schuler-Thurner, A. K. Palucka, and G. Schuler. 2001. Dendritic cells as vectors for therapy. Cell 106:271-274. - PubMed
-
- Becker, Y. 1995. An analysis of the role of skin Langerhans cells (LC) in the cytoplasmic processing of HIV-1 peptides after “peplotion” transepidermal transfer and HLA class I presentation to CD8+ CTLs—an approach to immunization of humans. Virus Genes 9:133-147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

