Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 RTA reactivates murine gammaherpesvirus 68 from latency
- PMID: 15709045
- PMCID: PMC548426
- DOI: 10.1128/JVI.79.5.3217-3222.2005
Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 RTA reactivates murine gammaherpesvirus 68 from latency
Abstract
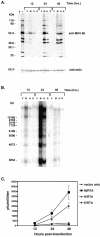
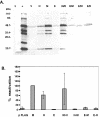
Murine gammaherpesvirus 68 (MHV-68), Kaposi's sarcoma-associated herpesvirus (HHV-8), and Epstein-Barr virus (EBV) are all members of the gammaherpesvirus family, characterized by their ability to establish latency in lymphocytes. The RTA protein, conserved in all gammaherpesviruses, is known to play a critical role in reactivation from latency. Here we report that HHV-8 RTA, not EBV RTA, was able to induce MHV-68 lytic viral proteins and DNA replication and processing and produce viable MHV-68 virions from latently infected cells at levels similar to those for MHV-68 RTA. HHV-8 RTA was also able to activate two MHV-68 lytic promoters, whereas EBV RTA was not. In order to define the domains of RTA responsible for their functional differences in viral promoter activation and initiation of the MHV-68 lytic cycle, chimeric RTA proteins were constructed by exchanging the N-terminal and C-terminal domains of the RTA proteins. Our data suggest that the species specificity of MHV-68 RTA resides in the N-terminal DNA binding domain.
Figures



Similar articles
-
Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency.J Virol. 2000 Apr;74(8):3659-67. doi: 10.1128/jvi.74.8.3659-3667.2000. J Virol. 2000. PMID: 10729142 Free PMC article.
-
The Rta/Orf50 transactivator proteins of the gamma-herpesviridae.Curr Top Microbiol Immunol. 2007;312:71-100. doi: 10.1007/978-3-540-34344-8_3. Curr Top Microbiol Immunol. 2007. PMID: 17089794 Review.
-
Function of Rta is essential for lytic replication of murine gammaherpesvirus 68.J Virol. 2001 Oct;75(19):9262-73. doi: 10.1128/JVI.75.19.9262-9273.2001. J Virol. 2001. PMID: 11533188 Free PMC article.
-
Alternatively initiated gene 50/RTA transcripts expressed during murine and human gammaherpesvirus reactivation from latency.J Virol. 2009 Jan;83(1):314-28. doi: 10.1128/JVI.01444-08. Epub 2008 Oct 29. J Virol. 2009. PMID: 18971285 Free PMC article.
-
Lytic cycle switches of oncogenic human gammaherpesviruses.Adv Cancer Res. 2007;97:81-109. doi: 10.1016/S0065-230X(06)97004-3. Adv Cancer Res. 2007. PMID: 17419942 Review.
Cited by
-
High-resolution functional profiling of a gammaherpesvirus RTA locus in the context of the viral genome.J Virol. 2009 Feb;83(4):1811-22. doi: 10.1128/JVI.02302-08. Epub 2008 Dec 10. J Virol. 2009. PMID: 19073723 Free PMC article.
-
The replication and transcription activator of murine gammaherpesvirus 68 cooperatively enhances cytokine-activated, STAT3-mediated gene expression.J Biol Chem. 2017 Sep 29;292(39):16257-16266. doi: 10.1074/jbc.M117.786970. Epub 2017 Aug 15. J Biol Chem. 2017. PMID: 28821622 Free PMC article.
-
Inhibition of NF-kappaB activation in vivo impairs establishment of gammaherpesvirus latency.PLoS Pathog. 2007 Jan;3(1):e11. doi: 10.1371/journal.ppat.0030011. PLoS Pathog. 2007. PMID: 17257062 Free PMC article.
-
RTA Occupancy of the Origin of Lytic Replication during Murine Gammaherpesvirus 68 Reactivation from B Cell Latency.Pathogens. 2017 Feb 16;6(1):9. doi: 10.3390/pathogens6010009. Pathogens. 2017. PMID: 28212352 Free PMC article.
-
Inhibition of the phosphatidylinositol 3-kinase-Akt pathway enhances gamma-2 herpesvirus lytic replication and facilitates reactivation from latency.J Gen Virol. 2010 Feb;91(Pt 2):463-9. doi: 10.1099/vir.0.015073-0. Epub 2009 Oct 28. J Gen Virol. 2010. PMID: 19864499 Free PMC article.
References
-
- Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. - PubMed
-
- Cesarman, E., and D. M. Knowles. 1997. Kaposi's sarcoma-associated herpesvirus: a lymphotropic human herpesvirus associated with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Semin. Diagn. Pathol. 14:54-66. - PubMed
-
- Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

