Diffusion-weighted imaging of acute excitotoxic brain injury
- PMID: 15709116
- PMCID: PMC7974100
Diffusion-weighted imaging of acute excitotoxic brain injury
Figures
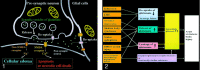

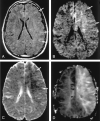
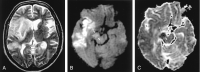
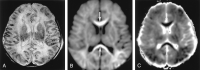
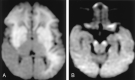


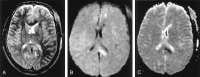
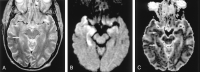
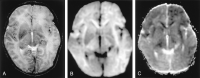
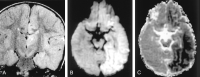
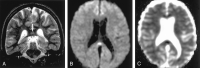
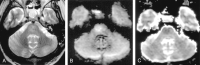
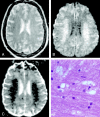
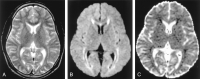
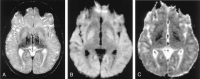
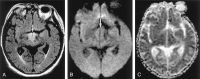
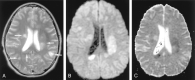

References
-
- Hassel B, Boudingh KA, Narvesen C, et al. Glutamate transport, glutamine synthase and phosphate-activated glutamimnase in rat CNS white matter. A quantitative study. J Neurochem 2003;87:230–237 - PubMed
-
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 1994;330:613–622 - PubMed
-
- Sharp FR, Swanson RA, Honkaniemi J, et al. Neurochemistry and molecular biology. In: Barnett HJM, Mohr JP, Stein BM, et al. Stroke Pathophysiology, Diagnosis, and Management. 1998;54–56
-
- Buchan AM, Slivka A, Xue D. The effect of the NMDA receptor antagonist MK-801 on cerebral blood flow and infarct volume in experimental focal stroke. Brain Res 1992;574:171–177 - PubMed
-
- Ogawa T, Okudera T, Inugami A, et al. Degeneration of the ipsilateral substantia nigra after striatal infarction: evaluation with MR imaging. Radiology 1997;204:847–851 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
