Arterial occlusion revealed by CT angiography predicts NIH stroke score and acute outcomes after IV tPA treatment
- PMID: 15709120
- PMCID: PMC7974096
Arterial occlusion revealed by CT angiography predicts NIH stroke score and acute outcomes after IV tPA treatment
Abstract
Background and purpose: The relationship between location of occlusion and clinical outcome is poorly understood in patients receiving intravenous tissue-type plasminogen activator (IV tPA). We postulated that acute stroke patients receiving IV tPA with patent vasculature or occult arterial occlusion by CT angiography (CTA) would have better outcomes and decreased hemorrhagic risk.
Methods: We identified 47 patients from our prospective stroke database who underwent CTA before treatment with IV tPA. Site of occlusion was categorized as M1 segment of the middle cerebral artery, M2 segment, multiple (either carotid, basilar, or both middle and anterior cerebral arteries), or absent (no occlusion proximal to M3). The effect of site of occlusion on National Institutes of Health Stroke Scale (NIHSS), early improvement (> or = 4-point improvement in NIHSS at 24 hours after treatment), intracranial hemorrhages, and modified Rankin scale (mRS) at 7 days was tested in a multivariate analysis.
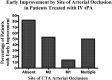
Results: The location of occlusion correlated with initial NIHSS for multiple, M1, M2 and absent occlusions (median NIHSS scores were 18, 18, 15, 10, respectively) (P < .02, rank sum). Following adjustment for initial NIHSS, age, and time to treatment, the absence of occlusion remained associated with early improvement (OR 5.0, 95% CI 1.1-23.3; P = .04) and independence at day 7 (mRS < or = 2) (OR 6.8, 95% CI 1.3-34.6; P = .02). Overall prevalence of symptomatic hemorrhages was 6.4%. Patients without occlusion had no hemorrhages (0% versus 23.3%; P < .04).
Conclusion: Among patients treated with tPA, those with patent vasculature or occult distal occlusion on CTA before treatment have lower NIHSS, better chances of early improvement and early independence with fewer hemorrhages.
Figures

References
-
- The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 - PubMed
-
- The NINDS t-PA stroke study group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118 - PubMed
-
- Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European cooperative acute stroke study (ECASS). JAMA. 1995;274:1017–1025 - PubMed
-
- Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian acute stroke study investigators. Lancet. 1998;352:1245–1251 - PubMed
-
- Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: A randomized controlled trial. JAMA. 1999;282:2019–2026 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
