Calmodulin is required for vasopressin-stimulated increase in cyclic AMP production in inner medullary collecting duct
- PMID: 15710610
- PMCID: PMC1182701
- DOI: 10.1074/jbc.M500040200
Calmodulin is required for vasopressin-stimulated increase in cyclic AMP production in inner medullary collecting duct
Abstract
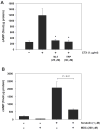
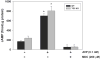
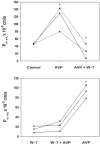
Calmodulin plays a critical role in regulation of renal collecting duct water permeability by vasopressin. However, specific targets for calmodulin action have not been thoroughly addressed. In the present study, we investigated whether Ca2+/calmodulin regulates adenylyl cyclase activity in the renal inner medullary collecting duct. Rat inner medullary collecting duct suspensions were incubated in the presence or absence of 0.1 nM vasopressin and the calmodulin inhibitors, monodansylcadaverine, W-7, and trifluoperazine, followed by measurement of cAMP. Vasopressin-stimulated cAMP elevation was significantly attenuated in the presence of calmodulin inhibitors. Analysis of transglutaminase 2 knock-out mice confirmed that these compounds were not acting through inhibition of transglutaminase 2 activity. Calmodulin inhibitors also blocked both cholera toxin- and forskolin-stimulated cAMP accumulation. In isolated perfused tubules, W-7 reversibly blocked vasopressin-stimulated urea permeability, a process that requires a rise in intracellular cAMP but does not appear to involve protein trafficking to the apical plasma membrane. These results suggest that calmodulin is required for vasopressin-stimulated adenylyl cyclase activity in the intact inner medullary collecting duct. Reverse transcription-PCR, immunoblotting, and immunohistochemistry revealed the presence of the calmodulin-sensitive adenylyl cyclase type 3 in the rat collecting duct, an isoform previously not known to be expressed in the collecting duct. Long-term treatment of Brattleboro rats with a vasopressin analog markedly decreased adenylyl cyclase type 3 protein abundance, providing an explanation for long-term down-regulation of vasopressin response in the collecting duct. These studies demonstrate the importance of calmodulin in the regulation of collecting duct adenylyl cyclase activity and transport function.
Figures





Similar articles
-
Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin.J Biol Chem. 2000 Nov 24;275(47):36839-46. doi: 10.1074/jbc.M005552200. J Biol Chem. 2000. PMID: 10973964
-
Characterization of vasopressin-responsive collecting duct adenylyl cyclases in the mouse.Am J Physiol Renal Physiol. 2010 Apr;298(4):F859-67. doi: 10.1152/ajprenal.00109.2009. Epub 2009 Dec 2. Am J Physiol Renal Physiol. 2010. PMID: 19955190 Free PMC article.
-
AVP-sensitive cAMP production is dependent on calmodulin in both MTAL and MCT.Am J Physiol. 1988 Nov;255(5 Pt 2):F834-40. doi: 10.1152/ajprenal.1988.255.5.F834. Am J Physiol. 1988. PMID: 2847548
-
Calcium signaling in vasopressin-induced aquaporin-2 trafficking.Pflugers Arch. 2008 Jul;456(4):747-54. doi: 10.1007/s00424-007-0371-7. Epub 2007 Oct 24. Pflugers Arch. 2008. PMID: 17957381 Review.
-
Spatial organisation of AKAP18 and PDE4 isoforms in renal collecting duct principal cells.Eur J Cell Biol. 2006 Jul;85(7):673-8. doi: 10.1016/j.ejcb.2006.01.005. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16500722 Review.
Cited by
-
Altered collecting duct adenylyl cyclase content in collecting duct endothelin-1 knockout mice.BMC Nephrol. 2007 May 23;8:8. doi: 10.1186/1471-2369-8-8. BMC Nephrol. 2007. PMID: 17521429 Free PMC article.
-
Regulation of nephron water and electrolyte transport by adenylyl cyclases.Am J Physiol Renal Physiol. 2014 Apr 1;306(7):F701-9. doi: 10.1152/ajprenal.00656.2013. Epub 2014 Jan 29. Am J Physiol Renal Physiol. 2014. PMID: 24477683 Free PMC article. Review.
-
Activation of AQP2 water channels without vasopressin: therapeutic strategies for congenital nephrogenic diabetes insipidus.Clin Exp Nephrol. 2018 Jun;22(3):501-507. doi: 10.1007/s10157-018-1544-8. Epub 2018 Feb 24. Clin Exp Nephrol. 2018. PMID: 29478202 Free PMC article. Review.
-
Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites.Proc Natl Acad Sci U S A. 2006 May 2;103(18):7159-64. doi: 10.1073/pnas.0600895103. Epub 2006 Apr 25. Proc Natl Acad Sci U S A. 2006. PMID: 16641100 Free PMC article.
-
Aquaporin-2 regulation in health and disease.Vet Clin Pathol. 2012 Dec;41(4):455-70. doi: 10.1111/j.1939-165x.2012.00488.x. Epub 2012 Nov 6. Vet Clin Pathol. 2012. PMID: 23130944 Free PMC article. Review.
References
-
- Knepper MA, Nielsen S, Chou CL, DiGiovanni SR. Semin. Nephrol. 1994;14:302–321. - PubMed
-
- Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA. J. Biol. Chem. 2000;275:36839–36846. - PubMed
-
- Chou CL, Christensen BM, Frische S, Vorum H, Desai RA, Hoffert JD, de LP, Nielsen S, Knepper MA. J. Biol. Chem. 2004;279:49026–49035. - PubMed
-
- Grantham JJ, Burg MB. Am. J. Physiol. 1966;211:255–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

