Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization
- PMID: 15710653
- PMCID: PMC2213050
- DOI: 10.1084/jem.20042060
Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization
Abstract
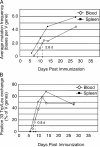
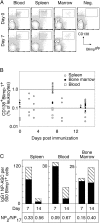
Immunization with a T cell-dependent antigen elicits production of specific memory B cells and antibody-secreting cells (ASCs). The kinetic and developmental relationships between these populations and the phenotypic forms they and their precursors may take remain unclear. Therefore, we examined the early stages of a primary immune response, focusing on the appearance of antigen-specific B cells in blood. Within 1 wk, antigen-specific B cells appear in the blood with either a memory phenotype or as immunoglobulin (Ig)G1 ASCs expressing blimp-1. The memory cells have mutated V(H) genes; respond to the chemokine CXCL13 but not CXCL12, suggesting recirculation to secondary lymphoid organs; uniformly express B220; show limited differentiation potential unless stimulated by antigen; and develop independently of blimp-1 expression. The antigen-specific IgG1 ASCs in blood show affinity maturation paralleling that of bone marrow ASCs, raising the possibility that this compartment is established directly by blood-borne ASCs. We find no evidence for a blimp-1-expressing preplasma memory compartment, suggesting germinal center output is restricted to ASCs and B220(+) memory B cells, and this is sufficient to account for the process of affinity maturation.
Figures




Comment in
-
Selecting B cells and plasma cells to memory.J Exp Med. 2005 Feb 21;201(4):497-9. doi: 10.1084/jem.20050218. J Exp Med. 2005. PMID: 15728231 Free PMC article.
References
-
- Zinkernagel, R.M. 2000. What is missing in immunology to understand immunity? Nat. Immunol. 1:181–185. - PubMed
-
- Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature. 381:751–758. - PubMed
-
- Klein, U., K. Rajewsky, and R. Kuppers. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679–1689. - PMC - PubMed
-
- Baine, Y., and G.J. Thorbecke. 1982. Induction and persistence of local B cell memory in mice. J. Immunol. 128:639–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

