A critical role of helix 3-helix 5 interaction in steroid hormone receptor function
- PMID: 15710879
- PMCID: PMC549476
- DOI: 10.1073/pnas.0409663102
A critical role of helix 3-helix 5 interaction in steroid hormone receptor function
Abstract
The ligand-binding domains of steroid hormone receptors possess a conserved structure with 12 alpha-helices surrounding a central hydrophobic core. On agonist binding, a repositioned helix 12 forms a pocket with helix 3 (H3) and helix 5 (H5), where transcriptional coactivators bind. The precise molecular interactions responsible for activation of these receptors remain to be elucidated. We previously identified a H3-H5 interaction that permits progesterone-mediated activation of a mutant mineralocorticoid receptor. We were intrigued to note that the potential for such interaction is widely conserved in the nuclear receptor family, indicating a possible functional significance. Here, we demonstrate via transcriptional activation studies in cell culture that alteration of residues involved in H3-H5 interaction consistently produces a gain of function in steroid hormone receptors. These data suggest that H3-H5 interaction may function as a molecular switch regulating the activity of nuclear receptors and suggest this site as a general target for pharmacologic intervention. Furthermore, they reveal a general mechanism for the creation of nuclear receptors bearing increased activity, providing a potentially powerful tool for the study of physiologic pathways in vivo.
Figures

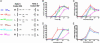
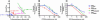

Similar articles
-
Differential interaction of RU486 with the progesterone and glucocorticoid receptors.J Mol Endocrinol. 2006 Aug;37(1):163-73. doi: 10.1677/jme.1.02089. J Mol Endocrinol. 2006. PMID: 16901932
-
The conserved residues of the ligand-binding domains of steroid receptors are located in the core of the molecules.J Mol Graph Model. 2001;19(6):543-51, 601-6. doi: 10.1016/s1093-3263(01)00087-0. J Mol Graph Model. 2001. PMID: 11552682
-
Analysis of the hormone-binding domain of steroid receptors using chimeras generated by homologous recombination.Exp Cell Res. 2005 Aug 15;308(2):320-33. doi: 10.1016/j.yexcr.2005.03.040. Exp Cell Res. 2005. PMID: 15936754
-
Helix 3-helix 5 interactions in steroid hormone receptor function.J Steroid Biochem Mol Biol. 2008 Apr;109(3-5):279-85. doi: 10.1016/j.jsbmb.2008.03.018. Epub 2008 Mar 13. J Steroid Biochem Mol Biol. 2008. PMID: 18502379 Free PMC article. Review.
-
Phasing the intranuclear organization of steroid hormone receptors.Biochem J. 2021 Jan 29;478(2):443-461. doi: 10.1042/BCJ20200883. Biochem J. 2021. PMID: 33512446 Review.
Cited by
-
Ligand-induced shifts in conformational ensembles that describe transcriptional activation.Elife. 2022 Oct 12;11:e80140. doi: 10.7554/eLife.80140. Elife. 2022. PMID: 36222302 Free PMC article.
-
A substitution in the ligand binding domain of the porcine glucocorticoid receptor affects activity of the adrenal gland.PLoS One. 2012;7(9):e45518. doi: 10.1371/journal.pone.0045518. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23029068 Free PMC article.
-
Characterization of a novel gain of function glucocorticoid receptor knock-in mouse.J Biol Chem. 2009 Mar 6;284(10):6249-59. doi: 10.1074/jbc.M807997200. Epub 2008 Nov 18. J Biol Chem. 2009. PMID: 19017639 Free PMC article.
-
Vestigialization of an allosteric switch: genetic and structural mechanisms for the evolution of constitutive activity in a steroid hormone receptor.PLoS Genet. 2014 Jan;10(1):e1004058. doi: 10.1371/journal.pgen.1004058. Epub 2014 Jan 9. PLoS Genet. 2014. PMID: 24415950 Free PMC article.
-
Molecular evolution of the switch for progesterone and spironolactone from mineralocorticoid receptor agonist to antagonist.Proc Natl Acad Sci U S A. 2019 Sep 10;116(37):18578-18583. doi: 10.1073/pnas.1903172116. Epub 2019 Aug 22. Proc Natl Acad Sci U S A. 2019. PMID: 31439819 Free PMC article.
References
-
- Moras, D. & Gronemeyer, H. (1998) Curr. Opin. Cell Biol. 10, 384–391. - PubMed
-
- Vivat, V., Gofflo, D., Garcia, T., Wurtz, J. M., Bourguet, W., Philibert, D. & Gronemeyer, H. (1997) J. Mol. Endocrinol. 18, 147–160. - PubMed
-
- Poujol, N., Wurtz, J. M., Tahiri, B., Lumbroso, S., Nicolas, J. C., Moras, D. & Sultan, C. (2000) J. Biol. Chem. 275, 24022–24031. - PubMed
-
- Dey, R., Roychowdhury, P. & Mukherjee, C. (2001) Protein Eng. 14, 565–571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

