Ca2+ blinks: rapid nanoscopic store calcium signaling
- PMID: 15710901
- PMCID: PMC548797
- DOI: 10.1073/pnas.0500059102
Ca2+ blinks: rapid nanoscopic store calcium signaling
Abstract
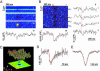
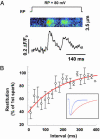
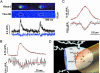
Luminal Ca(2+) in the endoplasmic and sarcoplasmic reticulum (ER/SR) plays an important role in regulating vital biological processes, including store-operated capacitative Ca(2+) entry, Ca(2+)-induced Ca(2+) release, and ER/SR stress-mediated cell death. We report rapid and substantial decreases in luminal [Ca(2+)], called "Ca(2+) blinks," within nanometer-sized stores (the junctional cisternae of the SR) during elementary Ca(2+) release events in heart cells. Blinks mirror small local increases in cytoplasmic Ca(2+),orCa(2+) sparks, but changes of [Ca(2+)] in the connected free SR network were below detection. Store microanatomy suggests that diffusional strictures may account for this paradox. Surprisingly, the nadir of the store depletion trails the peak of the spark by about 10 ms, and the refilling of local store occurs with a rate constant of 35 s(-1), which is approximately 6-fold faster than the recovery of local Ca(2+) release after a spark. These data suggest that both local store depletion and some time-dependent inhibitory mechanism contribute to spark termination and refractoriness. Visualization of local store Ca(2+) signaling thus broadens our understanding of cardiac store Ca(2+) regulation and function and opens the possibility for local regulation of diverse store-dependent functions.
Figures


References
-
- Neher, E. (1998) Neuron 20, 389-399. - PubMed
-
- Berridge, M. J., Bootman, M. D. & Roderick, H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517-529. - PubMed
-
- Bers, D. M. (2002) Nature 415, 198-205. - PubMed
-
- Clapham, D. E. (1995) Cell 80, 259-268. - PubMed
-
- Berridge, M. J. (2002) Cell Calcium 32, 235-249. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

