Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy
- PMID: 15711561
- PMCID: PMC1364464
- DOI: 10.1038/nm1190
Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy
Abstract
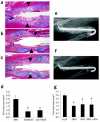
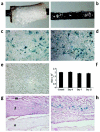
Structural allograft healing is limited because of a lack of vascularization and remodeling. To study this we developed a mouse model that recapitulates the clinical aspects of live autograft and processed allograft healing. Gene expression analyses showed that there is a substantial decrease in the genes encoding RANKL and VEGF during allograft healing. Loss-of-function studies showed that both factors are required for autograft healing. To determine whether addition of these signals could stimulate allograft vascularization and remodeling, we developed a new approach in which rAAV can be freeze-dried onto the cortical surface without losing infectivity. We show that combination rAAV-RANKL- and rAAV-VEGF-coated allografts show marked remodeling and vascularization, which leads to a new bone collar around the graft. In conclusion, we find that RANKL and VEGF are necessary and sufficient for efficient autograft remodeling and can be transferred using rAAV to revitalize structural allografts.
Figures





References
-
- Garbuz DS, Masri BA, Czitrom AA. Biology of allografting. Orthop. Clin. North. Am. 1998;29:199–204. - PubMed
-
- Goldberg VM, Stevenson S. The biology of bone grafts. Semin. Arthroplasty. 1993;4:58–63. - PubMed
-
- Einhorn TA. The cell and molecular biology of fracture healing. Clin. Orthop. 1998:S7–S21. - PubMed
-
- Burchardt H. Biology of bone transplantation. Orthop. Clin. North. Am. 1987;18:187–196. - PubMed
-
- Gould SE, Rhee JM, Tay B-B, Otsuka NY, Bradford DS. Cellular contribution of bone graft to fusion. J. Orthop. Res. 2000;18:920–927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR48149/AR/NIAMS NIH HHS/United States
- AR48681/AR/NIAMS NIH HHS/United States
- P01 HL051818/HL/NHLBI NIH HHS/United States
- R01 AR051469/AR/NIAMS NIH HHS/United States
- ES011854/ES/NIEHS NIH HHS/United States
- AR51469/AR/NIAMS NIH HHS/United States
- P01 HL066973/HL/NHLBI NIH HHS/United States
- R01 AR048149/AR/NIAMS NIH HHS/United States
- P01 ES011854/ES/NIEHS NIH HHS/United States
- P01 GM059299/GM/NIGMS NIH HHS/United States
- HL066973/HL/NHLBI NIH HHS/United States
- AR43510/AR/NIAMS NIH HHS/United States
- R01 AR048681/AR/NIAMS NIH HHS/United States
- R01 AR043510/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

