Epithelium-mesenchyme interactions control the activity of peroxisome proliferator-activated receptor beta/delta during hair follicle development
- PMID: 15713628
- PMCID: PMC549363
- DOI: 10.1128/MCB.25.5.1696-1712.2005
Epithelium-mesenchyme interactions control the activity of peroxisome proliferator-activated receptor beta/delta during hair follicle development
Abstract
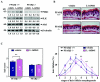
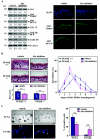
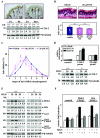
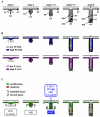
Hair follicle morphogenesis depends on a delicate balance between cell proliferation and apoptosis, which involves epithelium-mesenchyme interactions. We show that peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) and Akt1 are highly expressed in follicular keratinocytes throughout hair follicle development. Interestingly, PPARbeta/delta- and Akt1-deficient mice exhibit similar retardation of postnatal hair follicle morphogenesis, particularly at the hair peg stage, revealing a new important function for both factors in the growth of early hair follicles. We demonstrate that a time-regulated activation of the PPARbeta/delta protein in follicular keratinocytes involves the up-regulation of the cyclooxygenase 2 enzyme by a mesenchymal paracrine factor, the hepatocyte growth factor. Subsequent PPARbeta/delta-mediated temporal activation of the antiapoptotic Akt1 pathway in vivo protects keratinocytes from hair pegs against apoptosis, which is required for normal hair follicle development. Together, these results demonstrate that epithelium-mesenchyme interactions in the skin regulate the activity of PPARbeta/delta during hair follicle development via the control of ligand production and provide important new insights into the molecular biology of hair growth.
Figures






References
-
- Alonso, L. C., and R. L. Rosenfield. 2003. Molecular genetic and endocrine mechanisms of hair growth. Horm. Res. 60:1-13. - PubMed
-
- Billoni, N., B. Buan, B. Gautier, C. Collin, O. Gaillard, Y. F. Mahe, and B. A. Bernard. 2000. Expression of peroxisome proliferator activated receptors (PPARs) in human hair follicles and PPAR alpha involvement in hair growth. Acta Derm. Venereol. 80:329-334. - PubMed
-
- Botchkarev, V. A., N. V. Botchkareva, W. Roth, M. Nakamura, L. H. Chen, W. Herzog, G. Lindner, J. A. McMahon, C. Peters, R. Lauster, A. P. McMahon, and R. Paus. 1999. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat. Cell Biol. 1:158-164. - PubMed
-
- Braissant, O., and W. Wahli. 1998. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology 139:2748-2754. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
