Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity
- PMID: 15713843
- PMCID: PMC548950
- DOI: 10.1101/gad.320705
Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity
Abstract
Metaphase-to-anaphase transition is a fundamental step in cell cycle progression where duplicated sister-chromatids segregate to the future daughter cells. The anaphase-promoting complex/cyclosome (APC/C) is a highly regulated ubiquitin-ligase that triggers anaphase onset and mitotic exit by targeting securin and mitotic cyclins for destruction. It was previously shown that the Xenopus polo-like kinase Plx1 is essential to activate APC/C upon release from cytostatic factor (CSF) arrest in Xenopus egg extract. Although the mechanism by which Plx1 regulates APC/C activation remained unclear, the existence of a putative APC/C inhibitor was postulated whose activity would be neutralized by Plx1 upon CSF release. Here we identify XErp1, a novel Plx1-regulated inhibitor of APC/C activity, and we demonstrate that XErp1 is required to prevent anaphase onset in CSF-arrested Xenopus egg extract. Inactivation of XErp1 leads to premature APC/C activation. Conversely, addition of excess XErp1 to Xenopus egg extract prevents APC/C activation. Plx1 phosphorylates XErp1 in vitro at a site that targets XErp1 for degradation upon CSF release. Thus, our data lead to a model of APC/C activation in Xenopus egg extract in which Plx1 targets the APC/C inhibitor XErp1 for degradation.
Figures


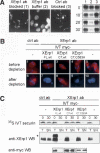

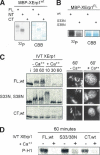
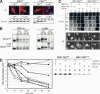
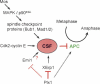
References
-
- Barr F.A., Sillje, H.H., and Nigg, E.A. 2004. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 5: 429–440. - PubMed
-
- Brassac T., Castro, A., Lorca, T., Le Peuch, C., Doree, M., Labbe, J.C., and Galas, S. 2000. The polo-like kinase Plx1 prevents premature inactivation of the APC(Fizzy)-dependent pathway in the early Xenopus cell cycle. Oncogene 19: 3782–3790. - PubMed
-
- Desai A., Murray, A., Mitchison, T.J., and Walczak, C.E. 1999. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61: 385–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
