NF-kappaB mediates proteolysis-inducing factor induced protein degradation and expression of the ubiquitin-proteasome system in skeletal muscle
- PMID: 15714207
- PMCID: PMC2361865
- DOI: 10.1038/sj.bjc.6602402
NF-kappaB mediates proteolysis-inducing factor induced protein degradation and expression of the ubiquitin-proteasome system in skeletal muscle
Abstract
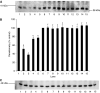
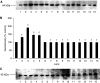
Loss of skeletal muscle in cancer cachexia has a negative effect on both morbidity and mortality. The role of nuclear factor-kappaB (NF-kappaB) in regulating muscle protein degradation and expression of the ubiquitin-proteasome proteolytic pathway in response to a tumour cachectic factor, proteolysis-inducing factor (PIF), has been studied by creating stable, transdominant-negative, muscle cell lines. Murine C(2)C(12) myoblasts were transfected with plasmids with a CMV promoter that had mutations at the serine phosphorylation sites required for degradation of I-kappaBalpha, an NF-kappaB inhibitory protein, and allowed to differentiate into myotubes. Proteolysis-inducing factor induced degradation of I-kappaBalpha, nuclear accumulation of NF-kappaB and an increase in luciferase reporter gene activity in myotubes containing wild-type, but not mutant, I-kappaBalpha proteins. Proteolysis-inducing factor also induced total protein degradation and loss of the myofibrillar protein myosin in myotubes containing wild-type, but not mutant, plasmids at the same concentrations as those causing activation of NF-kappaB. Proteolysis-inducing factor also induced increased expression of the ubiquitin-proteasome pathway, as determined by 'chymotrypsin-like' enzyme activity, the predominant proteolytic activity of the beta-subunits of the proteasome, protein expression of 20S alpha-subunits and the 19S subunits MSS1 and p42, as well as the ubiquitin conjugating enzyme, E2(14k), in cells containing wild-type, but not mutant, I-kappaBalpha. The ability of mutant I-kappaBalpha to inhibit PIF-induced protein degradation, as well as expression of the ubiquitin-proteasome pathway, confirms that both of these responses depend on initiation of transcription by NF-kappaB.
Figures








Similar articles
-
Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kappaB activation.Br J Cancer. 2004 Nov 1;91(9):1742-50. doi: 10.1038/sj.bjc.6602165. Br J Cancer. 2004. PMID: 15477867 Free PMC article.
-
Role of protein kinase C and NF-kappaB in proteolysis-inducing factor-induced proteasome expression in C(2)C(12) myotubes.Br J Cancer. 2004 May 4;90(9):1850-7. doi: 10.1038/sj.bjc.6601767. Br J Cancer. 2004. PMID: 15150589 Free PMC article.
-
Increased expression of the ubiquitin-proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-kappaB.Br J Cancer. 2003 Sep 15;89(6):1116-22. doi: 10.1038/sj.bjc.6601132. Br J Cancer. 2003. PMID: 12966435 Free PMC article.
-
The ubiquitin-proteasome pathway as a therapeutic target for muscle wasting.J Support Oncol. 2005 May-Jun;3(3):209-17. J Support Oncol. 2005. PMID: 15915823 Review.
-
Biochemical mechanisms of cellular catabolism.Curr Opin Clin Nutr Metab Care. 2002 Jul;5(4):401-5. doi: 10.1097/00075197-200207000-00009. Curr Opin Clin Nutr Metab Care. 2002. PMID: 12107376 Review.
Cited by
-
The molecular bases of training adaptation.Sports Med. 2007;37(9):737-63. doi: 10.2165/00007256-200737090-00001. Sports Med. 2007. PMID: 17722947 Review.
-
Chemotherapy-induced muscle wasting: association with NF-κB and cancer cachexia.Eur J Transl Myol. 2018 Jun 6;28(2):7590. doi: 10.4081/ejtm.2018.7590. eCollection 2018 Apr 24. Eur J Transl Myol. 2018. PMID: 29991992 Free PMC article.
-
Mechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: evidence from experimental and clinical studies.Eur J Nutr. 2013 Aug;52(5):1421-42. doi: 10.1007/s00394-013-0511-0. Epub 2013 Mar 19. Eur J Nutr. 2013. PMID: 23508457 Review.
-
Cancer cachexia: impact, mechanisms and emerging treatments.J Cachexia Sarcopenia Muscle. 2013 Jun;4(2):95-109. doi: 10.1007/s13539-012-0087-1. Epub 2012 Oct 25. J Cachexia Sarcopenia Muscle. 2013. PMID: 23097000 Free PMC article.
-
Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity-a review.Nutrients. 2010 Dec;2(12):1212-1230. doi: 10.3390/nu2121212. Epub 2010 Dec 9. Nutrients. 2010. PMID: 22254005 Free PMC article. Review.
References
-
- Almawi WY, Melemedjian OK (2002) Negative regulation of nuclear factor-κB activation and function by glucocorticoids. J Mol Endocrinol 28: 69–78 - PubMed
-
- Attaix D, Aurousseau E, Combaret L, Kee A, Larbaud D, Ralliere C, Souveine B, Taillander D, Tilignac T (1998) Ubiquitin–proteasome dependent proteolysis in skeletal muscle. Reprod Nutr Dev 38: 153–165 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

