Increasing stability of water-soluble PQQ glucose dehydrogenase by increasing hydrophobic interaction at dimeric interface
- PMID: 15715904
- PMCID: PMC551599
- DOI: 10.1186/1471-2091-6-1
Increasing stability of water-soluble PQQ glucose dehydrogenase by increasing hydrophobic interaction at dimeric interface
Abstract
Background: Water-soluble quinoprotein glucose dehydrogenase (PQQGDH-B) from Acinetobacter calcoaceticus has a great potential for application as a glucose sensor constituent. Because this enzyme shows no activity in its monomeric form, correct quaternary structure is essential for the formation of active enzyme. We have previously reported on the increasing of the stability of PQQGDH-B by preventing the subunit dissociation. Previous studies were based on decreasing the entropy of quaternary structure dissociation but not on increasing the interaction between the two subunits. We therefore attempted to introduce a hydrophobic interaction in the dimeric interface to increase the stability of PQQGDH-B.
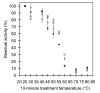
Results: Amino acid residues Asn340 and Tyr418 face each other at the dimer interface of PQQGDH-B, however no interaction exists between their side chains. We simultaneously substituted Asn340 to Phe and Tyr418 to Phe or Ile, to create the two mutants Asn340Phe/Tyr418Phe and Asn340Phe/Tyr418Ile. Furthermore, residues Leu280, Val282 and Val342 form a hydrophobic region that faces, on the other subunit, residues Thr416 and Thr417, again without any specific interaction. We simultaneously substituted Thr416 and Thr417 to Val, to create the mutant Thr416Val/Thr417Val. The temperatures resulting in lose of half of the initial activity of the constructed mutants were increased by 3-4 degrees C higher over wild type. All mutants showed 2-fold higher thermal stability at 55 degrees C than the wild-type enzyme, without decreasing their catalytic activities. From the 3D models of all the mutant enzymes, the predicted binding energies were found to be significantly greater that in the wild-type enzyme, consistent with the increases in thermal stabilities.
Conclusions: We have achieved via site-directed mutagenesis the improvement of the thermal stability of PQQGDH-B by increasing the dimer interface interaction. Through rational design based on the quaternary structure of the enzyme, we selected residues located at the dimer interface that do not contribute to the intersubunit interaction. By substituting these residues to hydrophobic ones, the thermal stability of PQQGDH-B was increased without decreasing its catalytic activity.
Figures




Similar articles
-
Stabilization of quaternary structure of water-soluble quinoprotein glucose dehydrogenase.Mol Biotechnol. 2003 Jun;24(2):97-104. doi: 10.1385/MB:24:2:97. Mol Biotechnol. 2003. PMID: 12746550
-
Engineering a chimeric pyrroloquinoline quinone glucose dehydrogenase: improvement of EDTA tolerance, thermal stability and substrate specificity.Protein Eng. 1999 Jan;12(1):63-70. doi: 10.1093/protein/12.1.63. Protein Eng. 1999. PMID: 10065712
-
Construction and characterization of mutant water-soluble PQQ glucose dehydrogenases with altered K(m) values--site-directed mutagenesis studies on the putative active site.Biochem Biophys Res Commun. 1999 Nov 2;264(3):820-4. doi: 10.1006/bbrc.1999.1157. Biochem Biophys Res Commun. 1999. PMID: 10544015
-
Cooperative effect of two surface amino acid mutations (Q252L and E170K) in glucose dehydrogenase from Bacillus megaterium IWG3 on stabilization of its oligomeric state.Appl Environ Microbiol. 2005 Jun;71(6):3285-93. doi: 10.1128/AEM.71.6.3285-3293.2005. Appl Environ Microbiol. 2005. PMID: 15933031 Free PMC article.
-
Molecular engineering of PQQGDH and its applications.Arch Biochem Biophys. 2004 Aug 1;428(1):52-63. doi: 10.1016/j.abb.2004.06.001. Arch Biochem Biophys. 2004. PMID: 15234269 Review. No abstract available.
Cited by
-
Does Acinetobacter calcoaceticus glucose dehydrogenase produce self-damaging H2O2?Biosci Rep. 2024 May 29;44(5):BSR20240102. doi: 10.1042/BSR20240102. Biosci Rep. 2024. PMID: 38687614 Free PMC article.
-
Characterization and engineering of a novel pyrroloquinoline quinone dependent glucose dehydrogenase from Sorangium cellulosum So ce56.Mol Biotechnol. 2011 Mar;47(3):253-61. doi: 10.1007/s12033-010-9339-5. Mol Biotechnol. 2011. PMID: 20886312
-
In silico panning for a non-competitive peptide inhibitor.BMC Bioinformatics. 2007 Jan 12;8:11. doi: 10.1186/1471-2105-8-11. BMC Bioinformatics. 2007. PMID: 17222344 Free PMC article.
-
The hydrophobic cluster on the surface of protein is the key structural basis for the SDS-resistance of chondroitinase VhChlABC.Mar Life Sci Technol. 2023 Nov 20;6(1):93-101. doi: 10.1007/s42995-023-00201-1. eCollection 2024 Feb. Mar Life Sci Technol. 2023. PMID: 38433971 Free PMC article.
-
Improvement of thermal stability via outer-loop ion pair interaction of mutated T1 lipase from Geobacillus zalihae strain T1.Int J Mol Sci. 2012;13(1):943-960. doi: 10.3390/ijms13010943. Epub 2012 Jan 17. Int J Mol Sci. 2012. PMID: 22312296 Free PMC article.
References
-
- Yokoyama K, Sode K, Tamiya E, Karube I. Integrated biosensor for glucose and galactose. Anal Chim Acta. 1989;218:137–142. doi: 10.1016/S0003-2670(00)80291-3. - DOI
-
- Ye L, Hammerle M, Olsthoorn AJJ, Schuhmann W, Schmidt H-L, Duine JA, Heller A. High current density "wired" quinoprotein glucose dehydrogenase electrode. Anal Chem. 1993;65:238–241.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

