Contribution of gap junctional communication between tumor cells and astroglia to the invasion of the brain parenchyma by human glioblastomas
- PMID: 15715906
- PMCID: PMC553963
- DOI: 10.1186/1471-2121-6-7
Contribution of gap junctional communication between tumor cells and astroglia to the invasion of the brain parenchyma by human glioblastomas
Abstract
Background: Gliomas are "intraparenchymally metastatic" tumors, invading the brain in a non-destructive way that suggests cooperation between glioma cells and their environment. Recent studies using an engineered rodent C6 tumor cell line have pointed to mechanisms of invasion that involved gap junctional communication (GJC), with connexin 43 as a substrate. We explored whether this concept may have clinical relevance by analyzing the participation of GJC in human glioblastoma invasion.
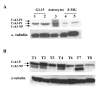
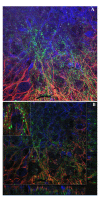
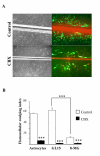
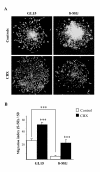
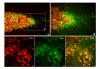
Results: Three complementary in vitro assays were used: (i) seeding on collagen IV, to analyze homocellular interactions between tumor cells (ii) co-cultures with astrocytes, to study glioblastoma/astrocytes relationships and (iii) implantation into organotypic brain slice cultures, that mimic the three-dimensional parenchymal environment. Carbenoxolone, a potent blocker of GJC, inhibited cell migration in the two latter models. It paradoxically increased it in the first one. These results showed that homocellular interaction between tumor cells supports intercellular adhesion, whereas heterocellular glioblastoma/astrocytes interactions through functional GJC conversely support tumor cell migration. As demonstrated for the rodent cell line, connexin 43 may be responsible for this heterocellular functional coupling. Its levels of expression, high in astrocytes, correlated positively with invasiveness in biopsied tumors.
Conclusions: our results underscore the potential clinical relevance of the concept put forward by other authors based on experiments with a rodent cell line, that glioblastoma cells use astrocytes as a substrate for their migration by subverting communication through connexin 43-dependent gap junctions.
Figures
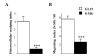
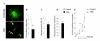

References
-
- Bernstein JJ. Local invasion and intraparenchymal metastasis of astrocytomas. Neuropathol Appl Neurobiol. 1996;22:421–424. - PubMed
-
- Reavey-Cantwell JF, Haroun RI, Zahurak M, Clatterbuck RE, Parker RJ, Mehta R, Fruehauf JP, Brem H. The prognostic value of tumor markers in patients with glioblastoma multiforme: analysis of 32 patients and review of the literature. J Neurooncol. 2001;55:195–204. doi: 10.1023/A:1013845004294. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

