Plasticity of B cell receptor internalization upon conditional depletion of clathrin
- PMID: 15716350
- PMCID: PMC1087239
- DOI: 10.1091/mbc.e05-01-0025
Plasticity of B cell receptor internalization upon conditional depletion of clathrin
Abstract
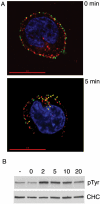
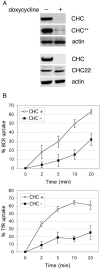
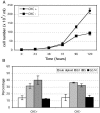
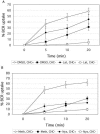
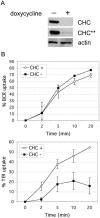
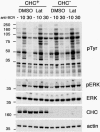
B cell antigen receptor (BCR) association with lipid rafts, the actin cytoskeleton, and clathrin-coated pits influences B cell signaling and antigen presentation. Although all three cellular structures have been separately implicated in BCR internalization, the relationship between them has not been clearly defined. In this study, internalization pathways were characterized by specifically blocking each potential mechanism of internalization. BCR uptake was reduced by approximately 70% in B cells conditionally deficient in clathrin heavy chain expression. Actin or raft antagonists were both able to block the residual, clathrin-independent BCR internalization. These agents also affected clathrin-dependent internalization, indicating that clathrin-coated pits, in concert with mechanisms dependent on rafts and actin, mediate the majority of BCR internalization. Clustering G(M1) gangliosides enhanced clathrin-independent BCR internalization, and this required actin. Thus, although rafts or actin independently did not mediate BCR internalization, they apparently cooperate to promote some internalization even in the absence of clathrin. Simultaneous inhibition of all BCR uptake pathways resulted in sustained tyrosine phosphorylation and activation of the extracellular signal-regulated kinase (ERK), strongly suggesting that downstream BCR signaling can occur without receptor translocation to endosomes and that internalization leads to signal attenuation.
Figures

References
-
- Brodsky, F. M., Chen, C. Y., Knuehl, C., Towler, M. C., and Wakeham, D. E. (2001). Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17, 517–568. - PubMed
-
- Brown, B. K., and Song, W. (2001). The actin cytoskeleton is required for the trafficking of the B cell antigen receptor to the late endosomes. Traffic 2, 414–427. - PubMed
-
- Buerstedde, J. M., and Takeda, S. (1991). Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell 67, 179–188. - PubMed
-
- Chen, C-Y. and Brodsky, F. M. (2005). Huntingtin-interacting protein 1 (Hip 1) and Hip 1-related protein (Hip 1R) bind the conserved sequences of clathrin light chains and thereby influence clathrin assembly in vitro and actin distribution in vivo. J. Biol. Chem. 280, 6109–6117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

