Temporal separation of replication and recombination requires the intra-S checkpoint
- PMID: 15716375
- PMCID: PMC2171758
- DOI: 10.1083/jcb.200410006
Temporal separation of replication and recombination requires the intra-S checkpoint
Abstract
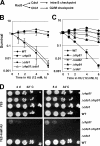
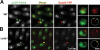
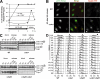
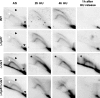
In response to DNA damage and replication pausing, eukaryotes activate checkpoint pathways that prevent genomic instability by coordinating cell cycle progression with DNA repair. The intra-S-phase checkpoint has been proposed to protect stalled replication forks from pathological rearrangements that could result from unscheduled recombination. On the other hand, recombination may be needed to cope with either stalled forks or double-strand breaks resulting from hydroxyurea treatment. We have exploited fission yeast to elucidate the relationship between replication fork stalling, loading of replication and recombination proteins onto DNA, and the intra-S checkpoint. Here, we show that a functional recombination machinery is not essential for recovery from replication fork arrest and instead can lead to nonfunctional fork structures. We find that Rad22-containing foci are rare in S-phase cells, but peak in G2 phase cells after a perturbed S phase. Importantly, we find that the intra-S checkpoint is necessary to avoid aberrant strand-exchange events during a hydroxyurea block.
Figures

References
-
- Brewer, B.J., and W.L. Fangman. 1987. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 51:463–471. - PubMed

