Linking axonal degeneration to microtubule remodeling by Spastin-mediated microtubule severing
- PMID: 15716377
- PMCID: PMC2171748
- DOI: 10.1083/jcb.200409058
Linking axonal degeneration to microtubule remodeling by Spastin-mediated microtubule severing
Abstract
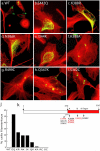
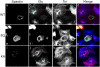
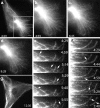
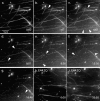
Mutations in the AAA adenosine triphosphatase (ATPase) Spastin (SPG4) cause an autosomal dominant form of hereditary spastic paraplegia, which is a retrograde axonopathy primarily characterized pathologically by the degeneration of long spinal neurons in the corticospinal tracts and the dorsal columns. Using recombinant Spastin, we find that six mutant forms of Spastin, including three disease-associated forms, are severely impaired in ATPase activity. In contrast to a mutation designed to prevent adenosine triphosphate (ATP) binding, an ATP hydrolysis-deficient Spastin mutant predicted to remain kinetically trapped on target proteins decorates microtubules in transfected cells. Analysis of disease-associated missense mutations shows that some more closely resemble the canonical hydrolysis mutant, whereas others resemble the ATP-binding mutant. Using real-time imaging, we show that Spastin severs microtubules when added to permeabilized, cytosol-depleted cells stably expressing GFP-tubulin. Using purified components, we also show that Spastin interacts directly with microtubules and is sufficient for severing. These studies suggest that defects in microtubule severing are a cause of axonal degeneration in human disease.
Figures

References
-
- Antonsson, B., D.B. Kassel, G. Di Paolo, R. Lutjens, B.M. Riederer, and G. Grenningloh. 1998. Identification of in vitro phosphorylation sites in the growth cone protein SCG10. Effect of phosphorylation site mutants on microtubule-destabilizing activity. J. Biol. Chem. 273:8439–8446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

