Gq/11-induced and spontaneous waves of coordinated network activation in developing frontal cortex
- PMID: 15716410
- PMCID: PMC6725933
- DOI: 10.1523/JNEUROSCI.2765-04.2005
Gq/11-induced and spontaneous waves of coordinated network activation in developing frontal cortex
Abstract
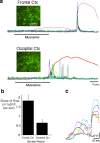
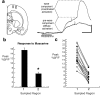
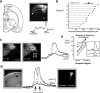
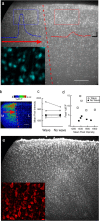
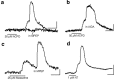
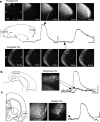
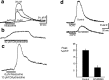
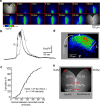
Repeated episodes of spontaneous large-scale neuronal bursting and calcium influx in the developing brain can potentially affect such fundamental processes as circuit formation and gene expression. Between postnatal day 3 (P3) and P7, the immature cortex can express one such form of activation whereby a wave of neuronal activity propagates through cortical networks, generating massive calcium influx. We previously showed that this activity could be triggered by brief stimulation of muscarinic receptors. Here, we show, by monitoring large cortical areas at low magnification, that although all areas respond to muscarinic agonists to some extent, only some areas are likely to generate the coordinated wave-like activation. The waves can be triggered repeatedly in frontal areas where, as we also show, waves occur spontaneously at a low frequency. In parietal and occipital areas, no such waves are seen. This selectivity may be related in part to differences in the cortical distribution of dopaminergic signaling, because we find that activation of dopamine receptors enables the response. Because M1 muscarinic receptors are typically coupled with G-alpha(q)/11, we investigated whether other receptors known to couple with this G-protein (group I glutamate metabotropic receptors, neurotensin type 1) could similarly elicit wave-like activation in responsive cortical areas. Our results suggest that multiple neurotransmitter systems can enable this form of activation in the frontal cortex. The findings suggest that a poorly recognized, developmentally regulated form of strong network activation found predominantly in the frontal cortex could potentially exert a profound influence on brain development.
Figures
Similar articles
-
Functional selectivity induced by mGlu₄ receptor positive allosteric modulation and concomitant activation of Gq coupled receptors.Neuropharmacology. 2013 Mar;66:122-32. doi: 10.1016/j.neuropharm.2012.03.003. Epub 2012 Mar 9. Neuropharmacology. 2013. PMID: 22426233 Free PMC article.
-
Involvement of betagamma subunits of G(q/11) in muscarinic M(1) receptor potentiation of corticotropin-releasing hormone-stimulated adenylyl cyclase activity in rat frontal cortex.J Neurochem. 2000 Jul;75(1):233-9. doi: 10.1046/j.1471-4159.2000.0750233.x. J Neurochem. 2000. PMID: 10854266
-
Stimulated D(1) dopamine receptors couple to multiple Galpha proteins in different brain regions.J Neurochem. 2001 Sep;78(5):981-90. doi: 10.1046/j.1471-4159.2001.00470.x. J Neurochem. 2001. PMID: 11553672
-
Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine.Trends Pharmacol Sci. 2007 Nov;28(11):551-5. doi: 10.1016/j.tips.2007.10.001. Epub 2007 Oct 22. Trends Pharmacol Sci. 2007. PMID: 17950471 Review.
-
Receptors as microprocessors: pharmacological nuance on metabotropic glutamate receptors 1alpha.Sci STKE. 2006 Jul 3;2006(342):pe29. doi: 10.1126/stke.3422006pe29. Sci STKE. 2006. PMID: 16818798 Review.
Cited by
-
Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo.J Neurosci. 2009 Jul 15;29(28):9011-25. doi: 10.1523/JNEUROSCI.5646-08.2009. J Neurosci. 2009. PMID: 19605639 Free PMC article.
-
Diversity of lysophosphatidic acid receptor-mediated intracellular calcium signaling in early cortical neurogenesis.J Neurosci. 2010 May 26;30(21):7300-9. doi: 10.1523/JNEUROSCI.6151-09.2010. J Neurosci. 2010. PMID: 20505096 Free PMC article.
-
Patterns of Spontaneous Local Network Activity in Developing Cerebral Cortex: Relationship to Adult Cognitive Function.PLoS One. 2015 Jun 22;10(6):e0131259. doi: 10.1371/journal.pone.0131259. eCollection 2015. PLoS One. 2015. PMID: 26098958 Free PMC article.
-
Prenatal activity from thalamic neurons governs the emergence of functional cortical maps in mice.Science. 2019 Jun 7;364(6444):987-990. doi: 10.1126/science.aav7617. Epub 2019 May 2. Science. 2019. PMID: 31048552 Free PMC article.
-
Voltage-sensitive dye imaging reveals dynamic spatiotemporal properties of cortical activity after spontaneous muscle twitches in the newborn rat.J Neurosci. 2012 Aug 8;32(32):10982-94. doi: 10.1523/JNEUROSCI.1322-12.2012. J Neurosci. 2012. PMID: 22875932 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
