Phospholipase D1-promoted release of tissue plasminogen activator facilitates neurite outgrowth
- PMID: 15716416
- PMCID: PMC6725938
- DOI: 10.1523/JNEUROSCI.4850-04.2005
Phospholipase D1-promoted release of tissue plasminogen activator facilitates neurite outgrowth
Abstract
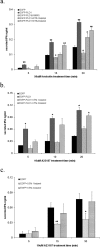
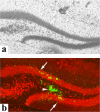
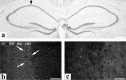
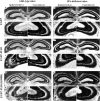
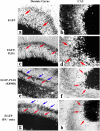
Temporal lobe epilepsy (TLE) is the most common form of epilepsy, affecting approximately 1-2% of the population. Seizure events resulting from TLE are characterized by aberrant hippocampal mossy fiber sprouting and plastic responses that affect brain function. Seizure susceptibility is modulated by the enzyme tissue plasminogen activator (tPA), the normal physiological role of which includes promotion of synaptic reorganization in the mossy fiber pathway by initiating a proteolytic cascade that cleaves extracellular matrix components and influences neurite extension. tPA is concentrated at and selectively secreted from growth cones during excitatory events. However, the mechanisms underlying tPA release during seizure-induced synaptogenesis are not well understood. We examine here potential roles for the signaling enzyme phospholipase D1 (PLD1), which promotes regulated exocytosis in non-CNS cell types, and which we previously demonstrated increases in expression in hippocampal neurons during seizure-induced mossy fiber sprouting. We now show that overexpression of wild-type PLD1 in cultured neurons promotes tPA release and tPA-dependent neurite extension, whereas overexpression of an inactive PLD1 allele or pharmacological inhibition of PLD1 inhibits tPA release. Similarly, viral delivery of wild-type PLD1 into the hippocampus facilitates tPA secretion and mossy fiber sprouting in a seizure-inducing model, whereas the inactive PLD1 allele inhibits tPA release and elicits blunted and abnormal mossy fiber extension similar to that observed for tPA-/- mice. Together, these findings secretion and thus mossy fiber extension in the setting of elevated suggest that PLD1 functions endogenously to regulate tPA-/- neuronal stimulation, such as that seen in TLE.
Figures


Similar articles
-
The tissue plasminogen activator (tPA)/plasmin extracellular proteolytic system regulates seizure-induced hippocampal mossy fiber outgrowth through a proteoglycan substrate.J Cell Biol. 2000 Mar 20;148(6):1295-304. doi: 10.1083/jcb.148.6.1295. J Cell Biol. 2000. PMID: 10725341 Free PMC article.
-
Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus.J Neurosci. 2002 Mar 15;22(6):2125-34. doi: 10.1523/JNEUROSCI.22-06-02125.2002. J Neurosci. 2002. PMID: 11896152 Free PMC article.
-
Increased expression of two phospholipase D isoforms during experimentally induced hippocampal mossy fiber outgrowth.Glia. 2004 Apr 1;46(1):74-83. doi: 10.1002/glia.10322. Glia. 2004. PMID: 14999815
-
Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system.Prog Brain Res. 2007;163:541-63. doi: 10.1016/S0079-6123(07)63029-5. Prog Brain Res. 2007. PMID: 17765737 Review.
-
The recurrent mossy fiber pathway of the epileptic brain.Neurochem Res. 2003 Nov;28(11):1649-58. doi: 10.1023/a:1026004904199. Neurochem Res. 2003. PMID: 14584819 Review.
Cited by
-
Transcriptional Upregulation of Plasminogen Activator Inhibitor-1 in Rat Primary Astrocytes by a Proteasomal Inhibitor MG132.Biomol Ther (Seoul). 2013 Mar;21(2):107-13. doi: 10.4062/biomolther.2012.102. Biomol Ther (Seoul). 2013. PMID: 24009867 Free PMC article.
-
Dopamine-induced plasticity, phospholipase D (PLD) activity and cocaine-cue behavior depend on PLD-linked metabotropic glutamate receptors in amygdala.PLoS One. 2011;6(9):e25639. doi: 10.1371/journal.pone.0025639. Epub 2011 Sep 27. PLoS One. 2011. PMID: 21980514 Free PMC article.
-
PLD1 Negatively Regulates Dendritic Branching.J Neurosci. 2012 Jun 6;32(23):7960-9. doi: 10.1523/JNEUROSCI.5378-11.2012. J Neurosci. 2012. PMID: 22674271 Free PMC article.
-
Microglial ablation and lipopolysaccharide preconditioning affects pilocarpine-induced seizures in mice.Neurobiol Dis. 2010 Jul;39(1):85-97. doi: 10.1016/j.nbd.2010.04.001. Epub 2010 Apr 9. Neurobiol Dis. 2010. PMID: 20382223 Free PMC article.
-
A novel approach for imaging brain-behavior relationships in mice reveals unexpected metabolic patterns during seizures in the absence of tissue plasminogen activator.Neuroimage. 2007 Oct 15;38(1):34-42. doi: 10.1016/j.neuroimage.2007.06.032. Epub 2007 Jul 18. Neuroimage. 2007. PMID: 17707126 Free PMC article.
References
-
- Attucci S, Torregrossa S, Moroni F, Giampietro D (2001) Metabotropic glutamate receptors stimulate phospholipase D via different pathways in the adult and neonate rat hippocampus. Neurochem Res 26: 1151-1155. - PubMed
-
- Baranes D, Lederfein D, Huang Y, Chen M, Bailey C, Kandel E (1998) Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron 21: 813-825. - PubMed
-
- Chen B, Lendvai B, Nimchinsky E, Burbach B, Fox K, Svoboda K (2000) Imaging high-resolution structure of GFP-expressing neurons in neocortex in vivo. Learn Mem 7: 433-441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
