Role of synaptic and intrinsic membrane properties in short-term receptive field dynamics in cat area 17
- PMID: 15716423
- PMCID: PMC6725929
- DOI: 10.1523/JNEUROSCI.3897-04.2005
Role of synaptic and intrinsic membrane properties in short-term receptive field dynamics in cat area 17
Abstract
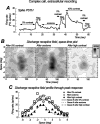
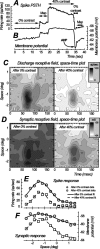
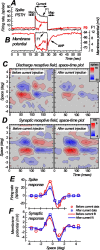
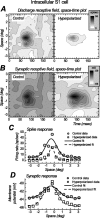
We examined the mechanisms through which the prolonged presentation of either a high-contrast stimulus or an artificial scotoma [equivalent to the stimulation of the receptive field (RF) surround] induces changes in the RF properties of neurons intracellularly recorded in cat primary visual cortex. Discharge and synaptic RFs were quantitatively characterized using bright and dark bars randomly flashed in various positions. Compared with the lack of stimulation (0% contrast for 15-30 s), stimulation with high-contrast sine-wave gratings (15-30 s) was followed by a strong reduction in gain and a weak but significant reduction in width of spike discharge RFs. These reductions were accompanied by a membrane potential hyperpolarization, a decrease of synaptic RF width, and varying changes of synaptic RF gain. Passive hyperpolarization by DC injection also produced significant reduction in the width and gain of discharge RF. Mimicking, in single neurons, high-contrast stimulation with high-intensity current injection also induced a membrane potential hyperpolarization, whose amplitude was correlated with discharge RF gain and width changes. Recovery from adaptation to high-contrast stimulation during the period of gray screen or scotoma presentation was associated with an increase in gain and discharge RF size. Stimulation of the RF surround with an artificial scotoma did not have any additional aftereffects over those of adaptation to a gray screen, indicating that the contraction and expansion of RF gain and size are attributable to intrinsic and synaptic mechanisms underlying adaptation and de-adaptation to strong visual stimuli.
Figures








Similar articles
-
Spatial dynamics of receptive fields in cat primary visual cortex related to the temporal structure of thalamocortical feedforward activity. Experiments and models.Exp Brain Res. 2002 Jun;144(4):430-44. doi: 10.1007/s00221-002-1061-5. Epub 2002 Apr 13. Exp Brain Res. 2002. PMID: 12037629
-
Membrane mechanisms underlying contrast adaptation in cat area 17 in vivo.J Neurosci. 2000 Jun 1;20(11):4267-85. doi: 10.1523/JNEUROSCI.20-11-04267.2000. J Neurosci. 2000. PMID: 10818163 Free PMC article.
-
Models of receptive-field dynamics in visual cortex.Vis Neurosci. 1999 Nov-Dec;16(6):1055-81. doi: 10.1017/s0952523899166070. Vis Neurosci. 1999. PMID: 10614587
-
Cortical plasticity revealed by circumscribed retinal lesions or artificial scotomas.Prog Brain Res. 2001;134:217-46. doi: 10.1016/s0079-6123(01)34016-5. Prog Brain Res. 2001. PMID: 11702546 Review.
-
Context, state and the receptive fields of striatal cortex cells.Trends Neurosci. 2000 Oct;23(10):497-503. doi: 10.1016/s0166-2236(00)01632-5. Trends Neurosci. 2000. PMID: 11006467 Review.
Cited by
-
Spatial and temporal features of synaptic to discharge receptive field transformation in cat area 17.J Neurophysiol. 2010 Feb;103(2):677-97. doi: 10.1152/jn.90946.2008. Epub 2009 Nov 11. J Neurophysiol. 2010. PMID: 19906874 Free PMC article.
-
Lack of orientation and direction selectivity in a subgroup of fast-spiking inhibitory interneurons: cellular and synaptic mechanisms and comparison with other electrophysiological cell types.Cereb Cortex. 2008 May;18(5):1058-78. doi: 10.1093/cercor/bhm137. Epub 2007 Aug 23. Cereb Cortex. 2008. PMID: 17720684 Free PMC article.
-
Cellular mechanisms underlying stimulus-dependent gain modulation in primary visual cortex neurons in vivo.Neuron. 2008 Jul 10;59(1):150-60. doi: 10.1016/j.neuron.2008.05.002. Neuron. 2008. PMID: 18614036 Free PMC article.
-
Effects of surround suppression on response adaptation of V1 neurons to visual stimuli.Dongwuxue Yanjiu. 2014 Sep;35(5):411-9. doi: 10.13918/j.issn.2095-8137.2014.5.411. Dongwuxue Yanjiu. 2014. PMID: 25297081 Free PMC article.
-
Dopamine modulation of phasing of activity in a rhythmic motor network: contribution of synaptic and intrinsic modulatory actions.J Neurophysiol. 2005 Nov;94(5):3101-11. doi: 10.1152/jn.00440.2005. Epub 2005 Jul 13. J Neurophysiol. 2005. PMID: 16014790 Free PMC article.
References
-
- Ahmed B, Allison JD, Douglas RJ, Martin KA (1997) An intracellular study of the contrast-dependence of neuronal activity in cat visual cortex. Cereb Cortex 7: 559-570. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
