Effect of ketamine on apoptosis by energy deprivation in astroglioma cells using flow cytometry system
- PMID: 15716615
- PMCID: PMC2808556
- DOI: 10.3346/jkms.2005.20.1.113
Effect of ketamine on apoptosis by energy deprivation in astroglioma cells using flow cytometry system
Abstract
Apoptosis is a programmed, physiologic mode of cell death that plays an important role in tissue homeostasis. As for the central nervous system, ischemic insults can induce pathophysiologic cascade of apoptosis in neurophils. Impairment of astrocyte functions during brain ischemia can critically influence neuron survival by neuronglia interactions. We aimed to elucidate the protective effect of ketamine on apoptosis by energy deprivation in astrocytes. Ischemic insults was induced with iodoacetate/ carbonylcyanide m-chlorophenylhydrazone (IAA/CCCP) 1.5 mM/20 microm or 150 microm/2 microm for 1 hr in the HTB-15 and CRL-1690 astrocytoma cells. Then these cells were reperfused with normal media or ketamine (0.1 mM) containing media for 1 hr or 24 hr. FITC-annexin-V staining and propidium iodide binding were determined by using flow cytometry. Cell size and granularity were measured by forward and side light scattering properties of flow cytometry system, respectively. An addition of ketamine during reperfusion increased the proportion of viable cells. Ketamine alleviated cell shrinkage and increased granularity during the early period, and ameliorated cell swelling during the late reperfusion period. Ketamine may have a valuable effect on amelioration of early and late apoptosis in the astrocytoma cells, even though the exact mechanism remains to be verified.
Figures
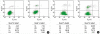

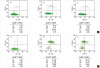
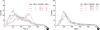
Similar articles
-
A flow cytometry assay simultaneously detects independent apoptotic parameters.Cytometry. 2001 Oct 1;45(2):151-7. doi: 10.1002/1097-0320(20011001)45:2<151::aid-cyto1157>3.0.co;2-i. Cytometry. 2001. PMID: 11590627
-
[Effects of ketamine on proliferation and apoptosis of pheochromocytoma cell].Fa Yi Xue Za Zhi. 2011 Dec;27(6):405-8, 412. Fa Yi Xue Za Zhi. 2011. PMID: 22393586 Chinese.
-
Ketamine modulates the stimulated adhesion molecule expression on human neutrophils in vitro.Anesth Analg. 2000 Jan;90(1):206-12. doi: 10.1097/00000539-200001000-00041. Anesth Analg. 2000. PMID: 10625005
-
Ketamine.Anaesthesia. 2007 Dec;62 Suppl 1:48-53. doi: 10.1111/j.1365-2044.2007.05298.x. Anaesthesia. 2007. PMID: 17937714 Review.
-
Ketamine and neurotoxicity: clinical perspectives and implications for emergency medicine.Ann Emerg Med. 2009 Aug;54(2):181-90. doi: 10.1016/j.annemergmed.2008.10.003. Epub 2008 Nov 6. Ann Emerg Med. 2009. PMID: 18990467 Review.
References
-
- Reno F, Burattini S, Rossi S, Luchetti F, Columbaro M, Santi S, Papa S, Falcieri E. Phospholipid rearrangement of apoptotic membrane does not depend on nuclear activity. Histochem Cell Biol. 1998;110:467–476. - PubMed
-
- Walton M, Sirimanne E, Reutelingsperger C, Williams C, Gluckman P, Dragunow M. Annexin V labels apoptotic neurons following hypoxia-ischemia. Neuroreport. 1997;8:3871–3875. - PubMed
-
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. - PMC - PubMed
-
- Rimon G, Bazenet CE, Philpott KL, Rubin LL. Increased surface phosphatidylserine is an early marker of neuronal apoptosis. J Neurosci Res. 1997;48:563–570. - PubMed
-
- van Engeland M, Ramaekers FC, Schutte B, Reutelingsperger CP. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry. 1996;24:131–139. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

