Neuronal death/survival signaling pathways in cerebral ischemia
- PMID: 15717004
- PMCID: PMC534909
- DOI: 10.1602/neurorx.1.1.17
Neuronal death/survival signaling pathways in cerebral ischemia
Abstract
Cumulative evidence suggests that apoptosis plays a pivotal role in cell death in vitro after hypoxia. Apoptotic cell death pathways have also been implicated in ischemic cerebral injury in in vivo ischemia models. Experimental ischemia and reperfusion models, such as transient focal/global ischemia in rodents, have been thoroughly studied and the numerous reports suggest the involvement of cell survival/death signaling pathways in the pathogenesis of apoptotic cell death in ischemic lesions. In these models, reoxygenation during reperfusion provides a substrate for numerous enzymatic oxidation reactions. Oxygen radicals damage cellular lipids, proteins and nucleic acids, and initiate cell signaling pathways after cerebral ischemia. Genetic manipulation of intrinsic antioxidants and factors in the signaling pathways has provided substantial understanding of the mechanisms involved in cell death/survival signaling pathways and the role of oxygen radicals in ischemic cerebral injury. Future studies of these pathways may provide novel therapeutic strategies in clinical stroke.
Figures
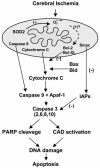
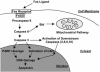

References
-
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21: 2–14, 2001. - PubMed
-
- Chan PH. Oxygen radicals in focal cerebral ischemia. Brain Pathol 4: 59–65, 1994. - PubMed
-
- Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma 17: 871–890, 2000. - PubMed
-
- Sugawara T, Lewen A, Gasche Y, Yu F, Chan PH. Overexpression of SOD1 protects vulnerable motor neurons after spinal cord injury by attenuating mitochondrial cytochrome c release. FASEB J 16: 1997–1999, 2002. - PubMed
-
- Liu R, Althaus JS, Ellerbrock BR, Becker DA, Gurney ME. Enhanced oxygen radical production in a transgenic mouse model of familial amyotrophic lateral sclerosis. Ann Neurol 44: 763–770, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

