Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults
- PMID: 15717010
- PMCID: PMC534915
- DOI: 10.1602/neurorx.1.1.101
Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults
Abstract
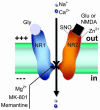
Excitotoxicity, defined as excessive exposure to the neurotransmitter glutamate or overstimulation of its membrane receptors, has been implicated as one of the key factors contributing to neuronal injury and death in a wide range of both acute and chronic neurologic disorders. Excitotoxic cell death is due, at least in part, to excessive activation of N-methyl-D-aspartate (NMDA)-type glutamate receptors and hence excessive Ca2+ influx through the receptor's associated ion channel. Physiological NMDA receptor activity, however, is also essential for normal neuronal function; potential neuroprotective agents that block virtually all NMDA receptor activity will very likely have unacceptable clinical side effects. For this reason many NMDA receptor antagonists have disappointingly failed advanced clinical trials for a number of diseases including stroke and neurodegenerative disorders such as Huntington's disease. In contrast, studies in my laboratory were the first to show that memantine, an adamantane derivative, preferentially blocks excessive NMDA receptor activity without disrupting normal activity. Memantine does this through its action as an open-channel blocker; it enters the receptor-associated ion channel preferentially when it is excessively open, and, most importantly, its off-rate is relatively fast so that it does not substantially accumulate in the channel to interfere with normal synaptic transmission. Past clinical use for other indications has demonstrated that memantine is well tolerated, and it has recently been approved in both Europe and the USA for the treatment of dementia of the Alzheimer's type. Clinical studies of the safety and efficacy of memantine for other neurological disorders, including glaucoma and other forms of dementia, are currently underway. A series of second-generation memantine derivatives are currently in development and may prove to have even greater neuroprotective properties than does memantine. These second-generation drugs take advantage of the fact that the NMDA receptor has other modulatory sites, in addition to its ion channel, that could potentially be used for safe but effective clinical intervention.
Figures




References
-
- Lipton SA, Rosenberg RA. Mechanisms of disease: excitatory amino acids as a final common pathway in neurologic disorders. N Engl J Med 330: 613–622, 1994. - PubMed
-
- Lipton SA, Nicotera P. Calcium, free radicals and excitotoxins in neuronal apoptosis. Cell Calcium 23: 165–171, 1998. - PubMed
-
- Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nature Neurosci 5[Suppl]: 1039–1042, 2002. - PubMed
-
- Kemp JA, Kew JN, Gill R. Handbook of experimental pharmacology, Vol 141 (Jonas P, Monyer H, eds), pp 495–527. Berlin: Springer, 1999.
-
- Lees KR, Asplund K, Carolei A, Davis SM, Diener HC, Kaste M et al. Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomized controlled trial. GAIN International Investigators. Lancet 355: 1949–1954, 2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

