Cellular transplantation strategies for spinal cord injury and translational neurobiology
- PMID: 15717046
- PMCID: PMC534951
- DOI: 10.1602/neurorx.1.4.424
Cellular transplantation strategies for spinal cord injury and translational neurobiology
Abstract
Basic science advances in spinal cord injury and regeneration research have led to a variety of novel experimental therapeutics designed to promote functionally effective axonal regrowth and sprouting. Among these interventions are cell-based approaches involving transplantation of neural and non-neural tissue elements that have potential for restoring damaged neural pathways or reconstructing intraspinal synaptic circuitries by either regeneration or neuronal/glial replacement. Notably, some of these strategies (e.g., grafts of peripheral nerve tissue, olfactory ensheathing glia, activated macrophages, marrow stromal cells, myelin-forming oligodendrocyte precursors or stem cells, and fetal spinal cord tissue) have already been translated to the clinical arena, whereas others have imminent likelihood of bench-to-bedside application. Although this progress has generated considerable enthusiasm about treating what once was thought to be a totally incurable condition, there are many issues to be considered relative to treatment safety and efficacy. The following review reflects on different experimental applications of intraspinal transplantation with consideration of the underlying pathological, pathophysiological, functional, and neuroplastic responses to spinal trauma that such treatments may target along with related issues of procedural and biological safety. The discussion then moves to an overview of ongoing and completed clinical trials to date. The pros and cons of these endeavors are considered, as well as what has been learned from them. Attention is primarily directed at preclinical animal modeling and the importance of patterning clinical trials, as much as possible, according to laboratory experiences.
Figures

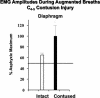




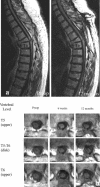
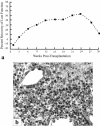
References
-
- Kao CC, Bunge RP, Reier PJ. Spinal cord reconstruction. New York: Raven Press, 1983.
-
- Sugar O, Gerard RW. Spinal cord regeneration in the rat. J Neurophysiol 3: 1–19, 1940.
-
- Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ 26: 238–255, 2002. - PubMed
-
- DeFelipe J, Jones EG. Cajal’s degeneration and regeneration of the nervous system (May RM, translator). New York: Oxford University Press, 1991.
-
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214: 931–933, 1981. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

