Brain-to-blood transporters for endogenous substrates and xenobiotics at the blood-brain barrier: an overview of biology and methodology
- PMID: 15717058
- PMCID: PMC539321
- DOI: 10.1602/neurorx.2.1.63
Brain-to-blood transporters for endogenous substrates and xenobiotics at the blood-brain barrier: an overview of biology and methodology
Abstract
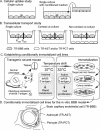
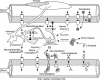
In the past decade, research into P-glycoprotein involving the blood-brain barrier (BBB) has seen a shift in the concept of the BBB as a structural barrier to that of a functional barrier for xenobiotics and changed simultaneously the strategy for the discovery and development of drugs acting in the CNS. As far as making advances in neurotherapeutics are concerned, the brain-to-blood transport function at the BBB will be one of the most important issues. Knowing the limitations of the in vivo and in vitro methods for BBB efflux research, it is essential to adopt a multidisciplinary approach in investigating the true physiological role of the BBB. Among several methods, the Brain Efflux Index method and the use of conditionally immortalized brain capillary endothelial cell lines, established from transgenic rats harboring temperature-sensitive simian virus 40 large T-antigen gene, are likely to be very useful tools for the BBB efflux transport research. According to our recent findings using these methods, several transporters in the brain capillary endothelial cells appear to play an important role in reducing the brain level of hydrophilic endogenous substrates produced either in the brain or peripheral organs, e.g., neurotransmitters, neuromodulators, metabolites of neurotransmitters, and uremic toxins. It has been reported also that large hydrophilic molecules, such as IgG, apo-transferrin, and amyloid-beta peptide, are susceptible to brain-to-blood efflux transport. In the light of the latest findings, we have formed the hypothesis that the BBB acts as a CNS detoxifying system for both endogenous substrates and xenobiotics in the brain. A fuller understanding of the physiological role of BBB efflux transporters will provide rational insights to assist in the development of safer neurotherapeutics.
Figures
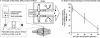
References
-
- Reese TS, Feder N, Brightman MW. Electron microscopic study of the blood-brain and blood-cerebrospinal fluid barriers with microperoxidase. J Neuropathol Exp Neurol 30: 137–138, 1971. - PubMed
-
- Tsuji A, Terasaki T, Takabatake Y, Tenda Y, Tamai I, Yamashima T, et al. P-glycoprotein as the drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci 51: 1427–1437, 1992. - PubMed
-
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77: 491–502, 1994. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

