Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda
- PMID: 15717259
- PMCID: PMC4515766
- DOI: 10.1086/427995
Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda
Abstract
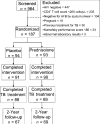
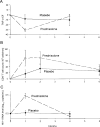
Background. Human immunodeficiency virus (HIV)-infected patients with tuberculosis (TB) respond to effective antituberculous therapy, but their prognosis remains poor. Mounting evidence from clinical studies supports the concept of copathogenesis in which immune activation that is triggered by TB and mediated by cytokines stimulates viral replication and worsens HIV infection, especially when immune function is preserved.Methods. We performed a phase 2, randomized, double-blind, placebo-controlled clinical trial in Kampala, Uganda, to determine whether immunoadjuvant prednisolone therapy in HIV-infected patients with TB who have CD4(+) T cell counts >/=200 cells/ mu L is safe and effective at increasing CD4(+) T cell counts.Results. Short-term prednisolone therapy reduced levels of immune activation and tended to produce higher CD4(+) T cell counts. Although prednisolone therapy was associated with a more rapid clearance of Mycobacterium tuberculosis from the sputum, it was also associated with a transient increase in HIV RNA levels, which receded when prednisolone therapy was discontinued. The intervention worsened underlying hypertension and caused fluid retention and hyperglycemia.Conclusion. The benefits of prednisolone therapy on immune activation and CD4(+) T cell counts do not outweigh the risks of adverse events in HIV-infected patients with TB and preserved immune function.
Figures

References
-
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. - PubMed
-
- Brindle R, Nunn P, Githui W, Allen BW, Gathua S, Waiyaki PG. Quantitative bacillary response to treatment in HIV-associated pulmonary tuberculosis. Am Rev Respir Dis. 1993;147:958–61. - PubMed
-
- Nunn P, Brindle R, Carpenter L, et al. Cohort study of human immunodeficiency virus infection in patients with tuberculosis in Nairobi, Kenya. Am Rev Respir Dis. 1992;146:849–54. - PubMed
-
- Chaisson RE, Schecter GF, Theuer CP, Rutherford GW, Echenberg DF, Hopewell PC. Tuberculosis in patients with the acquired immunodeficiency syndrome: clinical features, response to therapy, and survival. Am Rev Respir Dis. 1987;136:570–4. - PubMed
-
- Perriens JH, Colebunders RL, Karahunga C, et al. Increased mortality and tuberculosis treatment failure rate among human immunodeficiency virus seropositive compared to HIV seronegative patients with pulmonary tuberculosis treated with “standard” chemotherapy in Kinshasa, Zaire. Am Rev Respir Dis. 1991;144:750–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

