Maintenance of structure and function of mitochondrial Hsp70 chaperones requires the chaperone Hep1
- PMID: 15719019
- PMCID: PMC554129
- DOI: 10.1038/sj.emboj.7600580
Maintenance of structure and function of mitochondrial Hsp70 chaperones requires the chaperone Hep1
Abstract
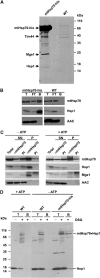
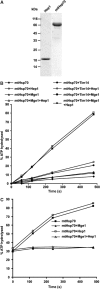
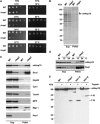
Hsp70 chaperones mediate folding of proteins and prevent their misfolding and aggregation. We report here on a new kind of Hsp70 interacting protein in mitochondria, Hep1. Hep1 is a highly conserved protein present in virtually all eukaryotes. Deletion of HEP1 results in a severe growth defect. Cells lacking Hep1 are deficient in processes that need the function of mitochondrial Hsp70s, such as preprotein import and biogenesis of proteins containing FeS clusters. In the mitochondria of these cells, Hsp70s, Ssc1 and Ssq1 accumulate as insoluble aggregates. We show that it is the nucleotide-free form of mtHsp70 that has a high tendency to self-aggregate. This process is efficiently counteracted by Hep1. We conclude that Hep1 acts as a chaperone that is necessary and sufficient to prevent self-aggregation and to thereby maintain the function of the mitochondrial Hsp70 chaperones.
Figures



Similar articles
-
Biogenesis of the mitochondrial Hsp70 chaperone.J Cell Biol. 2012 Oct 1;199(1):125-35. doi: 10.1083/jcb.201205012. Epub 2012 Sep 24. J Cell Biol. 2012. PMID: 23007651 Free PMC article.
-
Evolution of mitochondrial chaperones utilized in Fe-S cluster biogenesis.Curr Biol. 2006 Aug 22;16(16):1660-5. doi: 10.1016/j.cub.2006.06.069. Curr Biol. 2006. PMID: 16920629
-
Identification of a novel member of yeast mitochondrial Hsp70-associated motor and chaperone proteins that facilitates protein translocation across the inner membrane.FEBS Lett. 2005 Jan 17;579(2):507-11. doi: 10.1016/j.febslet.2004.12.018. FEBS Lett. 2005. PMID: 15642367
-
The control of spindle length by Hsp70 and Hsp110 molecular chaperones.FEBS Lett. 2013 Apr 17;587(8):1067-72. doi: 10.1016/j.febslet.2013.02.018. Epub 2013 Feb 19. FEBS Lett. 2013. PMID: 23434584 Review.
-
Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation.Crit Rev Biochem Mol Biol. 2007 Mar-Apr;42(2):95-111. doi: 10.1080/10409230701322298. Crit Rev Biochem Mol Biol. 2007. PMID: 17453917 Review.
Cited by
-
Thermal aggregates of human mortalin and Hsp70-1A behave as supramolecular assemblies.Int J Biol Macromol. 2020 Mar 1;146:320-331. doi: 10.1016/j.ijbiomac.2019.12.236. Epub 2019 Dec 30. Int J Biol Macromol. 2020. PMID: 31899237 Free PMC article.
-
Real-time observation of the conformational dynamics of mitochondrial Hsp70 by spFRET.EMBO J. 2013 May 29;32(11):1639-49. doi: 10.1038/emboj.2013.89. Epub 2013 Apr 26. EMBO J. 2013. PMID: 23624933 Free PMC article.
-
The Tom40 assembly process probed using the attachment of different intramitochondrial sorting signals.Mol Biol Cell. 2012 Oct;23(20):3936-47. doi: 10.1091/mbc.E12-03-0202. Epub 2012 Aug 29. Mol Biol Cell. 2012. PMID: 22933571 Free PMC article.
-
Human mitochondrial Hsp70 (mortalin): shedding light on ATPase activity, interaction with adenosine nucleotides, solution structure and domain organization.PLoS One. 2015 Jan 23;10(1):e0117170. doi: 10.1371/journal.pone.0117170. eCollection 2015. PLoS One. 2015. PMID: 25615450 Free PMC article.
-
Interactions of amyloidogenic proteins with mitochondrial protein import machinery in aging-related neurodegenerative diseases.Front Physiol. 2023 Nov 2;14:1263420. doi: 10.3389/fphys.2023.1263420. eCollection 2023. Front Physiol. 2023. PMID: 38028797 Free PMC article. Review.
References
-
- Angelidis CE, Lazaridis I, Pagoulatos GN (1999) Aggregation of hsp70 and hsc70 in vivo is distinct and temperature-dependent and their chaperone function is directly related to non-aggregated forms. Eur J Biochem 259: 505–512 - PubMed
-
- Azem A, Oppliger W, Lustig A, Jeno P, Feifel B, Schatz G, Horst M (1997) The mitochondrial hsp70 chaperone system. Effect of adenine nucleotides, peptide substrate, and mGrpE on the oligomeric state of mhsp70. J Biol Chem 272: 20901–20906 - PubMed
-
- Baumann F, Milisav I, Neupert W, Herrmann JM (2000) Ecm10, a novel hsp70 homolog in the mitochondrial matrix of the yeast Saccharomyces cerevisiae. FEBS Lett 487: 307–312 - PubMed
-
- Benaroudj N, Triniolles F, Ladjimi MM (1996) Effect of nucleotides, peptides, and unfolded proteins on the self-association of the molecular chaperone HSC70. J Biol Chem 271: 18471–18476 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

