Maintenance of structure and function of mitochondrial Hsp70 chaperones requires the chaperone Hep1
- PMID: 15719019
- PMCID: PMC554129
- DOI: 10.1038/sj.emboj.7600580
Maintenance of structure and function of mitochondrial Hsp70 chaperones requires the chaperone Hep1
Abstract
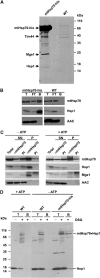
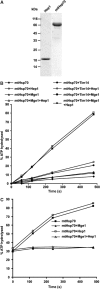
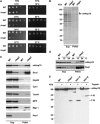
Hsp70 chaperones mediate folding of proteins and prevent their misfolding and aggregation. We report here on a new kind of Hsp70 interacting protein in mitochondria, Hep1. Hep1 is a highly conserved protein present in virtually all eukaryotes. Deletion of HEP1 results in a severe growth defect. Cells lacking Hep1 are deficient in processes that need the function of mitochondrial Hsp70s, such as preprotein import and biogenesis of proteins containing FeS clusters. In the mitochondria of these cells, Hsp70s, Ssc1 and Ssq1 accumulate as insoluble aggregates. We show that it is the nucleotide-free form of mtHsp70 that has a high tendency to self-aggregate. This process is efficiently counteracted by Hep1. We conclude that Hep1 acts as a chaperone that is necessary and sufficient to prevent self-aggregation and to thereby maintain the function of the mitochondrial Hsp70 chaperones.
Figures



References
-
- Angelidis CE, Lazaridis I, Pagoulatos GN (1999) Aggregation of hsp70 and hsc70 in vivo is distinct and temperature-dependent and their chaperone function is directly related to non-aggregated forms. Eur J Biochem 259: 505–512 - PubMed
-
- Azem A, Oppliger W, Lustig A, Jeno P, Feifel B, Schatz G, Horst M (1997) The mitochondrial hsp70 chaperone system. Effect of adenine nucleotides, peptide substrate, and mGrpE on the oligomeric state of mhsp70. J Biol Chem 272: 20901–20906 - PubMed
-
- Baumann F, Milisav I, Neupert W, Herrmann JM (2000) Ecm10, a novel hsp70 homolog in the mitochondrial matrix of the yeast Saccharomyces cerevisiae. FEBS Lett 487: 307–312 - PubMed
-
- Benaroudj N, Triniolles F, Ladjimi MM (1996) Effect of nucleotides, peptides, and unfolded proteins on the self-association of the molecular chaperone HSC70. J Biol Chem 271: 18471–18476 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

