Migration of polymorphonuclear leucocytes is influenced by dendritic cells
- PMID: 15720439
- PMCID: PMC1782099
- DOI: 10.1111/j.1365-2567.2005.02104.x
Migration of polymorphonuclear leucocytes is influenced by dendritic cells
Abstract
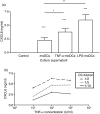
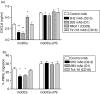
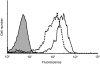
Dendritic cells (DCs) are the most potent antigen-presenting cells and populate many tissues where they may participate in inflammatory reactions. The infiltration of polymorphonuclear leucocytes (PMNLs) into tissues is a prominent feature of inflammation. The mechanisms of PMNL recruitment depend on chemotactic factors and adhesion molecules expressed on endothelial cells. The aim of the present study was to determine whether DCs participate in the early recruitment of PMNLs. Dendritic cells derived from peripheral blood monocytes were used for this study. PMNLs incubated with culture supernatant (CS) from untreated or from tumour necrosis factor-alpha (TNF-alpha)-treated (1 hr, 100 U/ml, 37 degrees ) monocyte-derived DCs (moDCs) had increased surface expression of both CD11b and CD18. Moreover, both untreated and TNF-alpha-treated moDCs induced PMNL chemotaxis. By blocking CXCL8, CXCL5, CXCL7 and Pan GRO (CXCL1, CXCL2, CXCL3), we observed that CXCL8/interleukin-8 might be the chemokine that induced the PMNL chemotactic activity in the CS of untreated and TNF-alpha-treated moDC. Furthermore, we investigated the regulation of CXCL8 production in moDCs by adhesion molecule engagement. Our data demonstrated that CD31, CD18, CD29 and CD49d participated in the adhesion of immature moDCs to endothelium. Moreover, engagement of domains 1-3 of CD31, but not of CD29 or CD18, decreased the production of CXCL8 by immature but not mature moDCs (which display lower CD31 levels than immature moDCs). Overall, these results suggest that DCs not only trigger a specific immune response, but also the innate immune response by recruiting PMNLs. Furthermore, our results also suggest that CXCL8 production by immature DCs might be regulated by signalling through CD31 during their migration through the vascular endothelium.
Figures



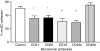


References
-
- Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990;70:427–51. - PubMed
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. - PubMed
-
- Mollinedo F, Borregaard N, Boxer LA. Novel trends in neutrophil structure, function and development. Immunol Today. 1999;20:535–7. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

