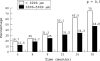Impact of lesion size on photodynamic therapy with verteporfin of predominantly classic lesions in age related macular degeneration
- PMID: 15722311
- PMCID: PMC1772570
- DOI: 10.1136/bjo.2004.050997
Impact of lesion size on photodynamic therapy with verteporfin of predominantly classic lesions in age related macular degeneration
Abstract
Aim: To determine if photodynamic therapy (PDT) outcomes are related to lesion size in patients with subfoveal predominantly classic choroidal neovascularisation (CNV) secondary to age related macular degeneration (AMD).
Methods: According to greatest linear dimension (GLD) of the entire lesion determined with fluorescein angiography (FA) patients were divided into two groups. In the first group GLD was <3000 microm and in the second one GLD was 3000-5000 microm. All eyes were treated with standard PDT with the verteporfin protocol. The primary outcome was the proportion of eyes in both groups that did not show significant leakage in FA at the end of follow up. Secondary outcomes were changes in GLD and in best corrected visual acuity (BCVA).
Results: 64 patients (mean (SD) age, 76.7 (7.7) years; range 58-95 years) were recruited to participate in the study. All participants in the study completed the follow up time (mean 16.6 months). 24 patients (75%) in the group of smaller lesions (n = 32) compared with 15 patients (46.8%) in the group of larger lesions (n = 32) did not show significant leakage in FA at the end of follow up (p = 0.02). A GLD increase >1000 microm was recorded in nine eyes (28.1%) in the group of smaller lesions and in 16 eyes (50%) in the group of larger lesions (p = 0.07). 22 eyes (68.7%) in the group of smaller lesions compared with 19 eyes (59.3%) in the group of larger lesions lost less than three lines of vision (p = 0.06). Relevant side effects related to verteporfin therapy were not recorded, except for four patients (6.2%) with infusion related back pain.
Conclusions: These results suggest that lesion size at baseline may be a prognosis factor in PDT in patients with subfoveal predominantly classic CNV secondary to AMD. There are no relevant side effects or safety concerns derived from verteporfin therapy.
Figures
References
-
- Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report 1. Arch Ophthalmol 1999;117:1329–45. - PubMed
-
- Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin. Two-year results of 2 randomized clinical trials—TAP Report 2. Arch Ophthalmol 2001;119:198–207. - PubMed
-
- Verteporfin in Photodynamic Therapy (VIP) Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: 2-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization—VIP Report 2. Am J Ophthalmol 2001;131:541–60. - PubMed
-
- Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in patients with age-related macular degeneration. Additional information regarding baseline lesion composition’s impact on vision outcomes—TAP report No 3. Arch Ophthalmol 2002;120:1443–54. - PubMed
-
- Verteporfin Roundtable 2000 and 2001 Participants. Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) Study Group Principal Investigators, and Verteporfin in Photodynamic Therapy (VIP) Study Group Principal Investigators. Guidelines for using verteporfin in photodynamic therapy to treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina 2002;22:6–18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical