The initial substrate-binding site of gamma-secretase is located on presenilin near the active site
- PMID: 15722417
- PMCID: PMC552920
- DOI: 10.1073/pnas.0407640102
The initial substrate-binding site of gamma-secretase is located on presenilin near the active site
Abstract
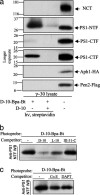
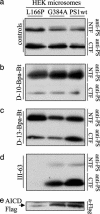
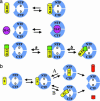
gamma-Secretase is a structurally enigmatic multiprotein complex that catalyzes intramembrane proteolysis of a variety of substrates, including the amyloid beta-protein precursor of Alzheimer's disease and the Notch receptor essential to cell differentiation. The active site of this transmembrane aspartyl protease apparently lies at the interface between two subunits of presenilin-1 (PS1); however, evidence suggests the existence of an initial substrate-binding site that is distinct from the active site. Here, we report that photoaffinity probes based on potent helical peptide inhibitors and designed to mimic the amyloid beta-protein precursor substrate bind specifically to the PS subunit interface, at a site close to the active site. The location of the helical peptide-binding site suggests that substrate passes between the two PS1 subunits to access the active site. An aggressive Alzheimer-causing mutation in PS1 strongly reduced photolabeling by a transition-state analogue but not by helical peptides, providing biochemical evidence that the pathological effect of this PS mutation is due to alteration of the active-site topography.
Figures
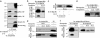
References
-
- Esler, W. P. & Wolfe, M. S. (2001) Science 293, 1449-1454. - PubMed
-
- De Strooper, B. (2003) Neuron 38, 9-12. - PubMed
-
- Wolfe, M. S. & Kopan, R. (2004) Science 305, 1119-1123. - PubMed
-
- Yu, G., Nishimura, M., Arawaka, S., Levitan, D., Zhang, L., Tandon, A., Song, Y. Q., Rogaeva, E., Chen, F., Kawarai, T., et al. (2000) Nature 407, 48-54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

