Identification of plasma membrane macro- and microdomains from wavelet analysis of FRET microscopy
- PMID: 15722423
- PMCID: PMC1305509
- DOI: 10.1529/biophysj.104.054056
Identification of plasma membrane macro- and microdomains from wavelet analysis of FRET microscopy
Abstract
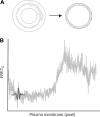
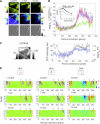
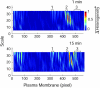
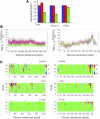
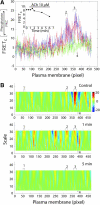
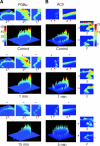
In this study, we sought to characterize functional signaling domains by applying the multiresolution properties of the continuous wavelet transform to fluorescence resonance energy transfer (FRET) microscopic images of plasma membranes. A genetically encoded FRET reporter of protein kinase C (PKC)-dependent phosphorylation was expressed in COS1 cells. Differences between wavelet coefficient matrices revealed several heterogeneous domains (typically ranging from 1 to 5 microm), reflecting the dynamic balance between PKC and phosphatase activity during stimulation with phorbol-12,13-dibutyrate or acetylcholine. The balance in these domains was not necessarily reflected in the overall plasma membrane changes, and observed heterogeneity was absent when cells were exposed to a phosphatase or PKC inhibitor. Prolonged exposure to phorbol-12,13-dibutyrate and acetylcholine yielded more homogeneous FRET distribution in plasma membranes. The proposed wavelet-based image analysis provides, for the first time, a basis and a means of detecting and quantifying dynamic changes in functional signaling domains, and may find broader application in studying fine aspects of cellular signaling by various imaging reporters.
Figures
Similar articles
-
Analysis of functional signaling domains from fluorescence imaging and the two-dimensional continuous wavelet transform.Biophys J. 2007 Oct 15;93(8):2900-10. doi: 10.1529/biophysj.106.102582. Epub 2007 Jun 22. Biophys J. 2007. PMID: 17586569 Free PMC article.
-
Genetically encoded FRET probe for PKC activity based on pleckstrin.J Am Chem Soc. 2004 Sep 29;126(38):11786-7. doi: 10.1021/ja0460155. J Am Chem Soc. 2004. PMID: 15382901
-
Imaging kinase--AKAP79--phosphatase scaffold complexes at the plasma membrane in living cells using FRET microscopy.J Cell Biol. 2003 Jan 6;160(1):101-12. doi: 10.1083/jcb.200209127. Epub 2002 Dec 30. J Cell Biol. 2003. PMID: 12507994 Free PMC article.
-
Pathway illuminated: visualizing protein kinase C signaling.IUBMB Life. 2003 Dec;55(12):653-60. doi: 10.1080/152165401310001642216. IUBMB Life. 2003. PMID: 14769001 Review.
-
Fanciful FRET.Sci STKE. 2006 Apr 18;2006(331):re2. doi: 10.1126/stke.3312006re2. Sci STKE. 2006. PMID: 16622184 Review.
Cited by
-
A guide to ERK dynamics, part 1: mechanisms and models.Biochem J. 2023 Dec 13;480(23):1887-1907. doi: 10.1042/BCJ20230276. Biochem J. 2023. PMID: 38038974 Free PMC article.
-
Cav1.2, cell proliferation, and new target in atherosclerosis.ISRN Biochem. 2013 May 12;2013:463527. doi: 10.1155/2013/463527. eCollection 2013. ISRN Biochem. 2013. PMID: 25937960 Free PMC article. Review.
-
Spatiotemporal analysis of endocytosis and membrane distribution of fluorescent sterols in living cells.Histochem Cell Biol. 2008 Nov;130(5):891-908. doi: 10.1007/s00418-008-0488-6. Epub 2008 Sep 12. Histochem Cell Biol. 2008. PMID: 18787836
-
Microdomain organization and frequency-dependence of CREB-dependent transcriptional signaling in heart cells.FASEB J. 2011 May;25(5):1544-55. doi: 10.1096/fj.10-176198. Epub 2011 Jan 19. FASEB J. 2011. PMID: 21248242 Free PMC article.
-
Heterogeneity of Calcium Channel/cAMP-Dependent Transcriptional Activation.Curr Mol Pharmacol. 2015;8(1):54-60. doi: 10.2174/1874467208666150507093601. Curr Mol Pharmacol. 2015. PMID: 25966705 Free PMC article. Review.
References
-
- Arneodo, A., E. Bacry, P. V. Graves, and J. F. Muzy. 1995. Characterizing long-range correlations in DNA sequences from wavelet analysis. Phys. Rev. Lett. 74:3293–3296. - PubMed
-
- Braz, J. C., K. Gregory, A. Pathak, W. Zhao, B. Sahin, R. Klevitsky, T. F. Kimball, J. N. Lorenz, A. C. Nairn, S. B. Liggett, I. Bodi, S. Wang, A. Schwartz, E. G. Lakatta, A. A. DePaoli-Roach, J. Robbins, T. E. Hewett, J. A. Bibb, M. V. Westfall, E. G. Kranias, and J. D. Molkentin. 2004. PKC-α regulates cardiac contractility and propensity toward heart failure. Nat. Med. 10:248–254. - PubMed
-
- Haralick, R. M., K. Shanmugam, and I. Dinstein. 1973. Textural features for image classification. IEEE Trans. 3:610–621.
-
- Kenworthy, A. K. 2001. Imaging protein-protein interactions using fluorescence resonance energy transfer microscopy. Methods. 24:289–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

