Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence
- PMID: 15722433
- PMCID: PMC1305515
- DOI: 10.1529/biophysj.104.045476
Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence
Abstract
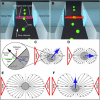
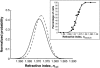
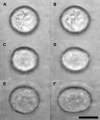
The relationship between the mechanical properties of cells and their molecular architecture has been the focus of extensive research for decades. The cytoskeleton, an internal polymer network, in particular determines a cell's mechanical strength and morphology. This cytoskeleton evolves during the normal differentiation of cells, is involved in many cellular functions, and is characteristically altered in many diseases, including cancer. Here we examine this hypothesized link between function and elasticity, enabling the distinction between different cells, by using a microfluidic optical stretcher, a two-beam laser trap optimized to serially deform single suspended cells by optically induced surface forces. In contrast to previous cell elasticity measurement techniques, statistically relevant numbers of single cells can be measured in rapid succession through microfluidic delivery, without any modification or contact. We find that optical deformability is sensitive enough to monitor the subtle changes during the progression of mouse fibroblasts and human breast epithelial cells from normal to cancerous and even metastatic state. The surprisingly low numbers of cells required for this distinction reflect the tight regulation of the cytoskeleton by the cell. This suggests using optical deformability as an inherent cell marker for basic cell biological investigation and diagnosis of disease.
Figures




References
-
- Aaronson, S. A., and G. J. Todaro. 1968a. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 162:1024–1026. - PubMed
-
- Aaronson, S. A., and G. J. Todaro. 1968b. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J. Cell. Physiol. 72:141–148. - PubMed
-
- Ashkin, A. 1970. Acceleration and trapping of particles by radiation pressure. Phys. Rev. Lett. 24:156–159.
-
- Ashkin, A., and J. M. Dziedzic. 1973. Radiation pressure on a free liquid surface. Phys. Rev. Lett. 30:139–142.
-
- Barer, R., and S. Joseph. 1954. Refractometry of living cells, Part I. Basic principles. Q. J. Microsc. Sci. 95:399–423.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

