Hsp20, a novel alpha-crystallin, prevents Abeta fibril formation and toxicity
- PMID: 15722443
- PMCID: PMC2279291
- DOI: 10.1110/ps.041020705
Hsp20, a novel alpha-crystallin, prevents Abeta fibril formation and toxicity
Abstract
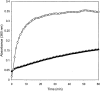
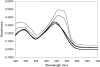
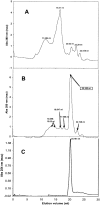
Beta-amyloid (Abeta) is a major protein component of senile plaques in Alzheimer's disease, and is neurotoxic when aggregated. The size of aggregated Abeta responsible for the observed neurotoxicity and the mechanism of aggregation are still under investigation; however, prevention of Abeta aggregation still holds promise as a means to reduce Abeta neurotoxicity. In research presented here, we show that Hsp20, a novel alpha-crystallin isolated from the bovine erythrocyte parasite Babesia bovis, was able to prevent aggregation of denatured alcohol dehydrogenase when the two proteins are present at near equimolar levels. We then examined the ability of Hsp20 produced as two different fusion proteins to prevent Abeta amyloid formation as indicated by Congo Red binding; we found that not only was Hsp20 able to dramatically reduce Congo Red binding, but it was able to do so at molar ratios of Hsp20 to Abeta of 1 to 1000. Electron microscopy confirmed that Hsp20 does prevent Abeta fibril formation. Hsp20 was also able to significantly reduce Abeta toxicity to both SH-SY5Y and PC12 neuronal cells at similar molar ratios. At high concentrations of Hsp20, the protein no longer displays its aggregation inhibition and toxicity attenuation properties. Size exclusion chromatography indicated that Hsp20 was active at low concentrations in which dimer was present. Loss of activity at high concentrations was associated with the presence of higher oligomers of Hsp20. This work could contribute to the development of a novel aggregation inhibitor for prevention of Abeta toxicity.
Figures





References
-
- Brown, W.C., Ruef, B.J., Norimine, J., Kegerreis, K.A., Suarez, C.E., Conley, P.G., Stich, R.W., Carson, K.H., and Rice-Ficht, A.C. 2001. A novel 20-kDa protein conserved in Babesia bovis and Babesia bigemina stimulates memory CD4+ T lymphocytes responses in B. bovis-immune cattle. Mol. Biochem. Parasitol. 118 97–109. - PubMed
-
- Bruey, J.M., Ducasse, C., Bonniaud, P., Ravagnan, L., Susin, S.A., Diaz-Latoud, C., Gurbuxani, S., Arrigo, A.P., Kroemer, G., Solary, E., et al. 2000. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2 645–652. - PubMed
-
- Chromy B.A., Nowak R.J., Lambert M.P., Viola K.L., Chang L., Velasco P.T., Jones B.W., Fernandez S.J., Lacor P.N., Horowitz P., Finch C.E., Krafft G.A., and Klein W.L. 2003. Self-assembly of Aβ(1–42) into globular neurotoxins. Biochemistry 42 12749–12760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

