Resetting of peripheral circadian clock by prostaglandin E2
- PMID: 15723041
- PMCID: PMC1299266
- DOI: 10.1038/sj.embor.7400356
Resetting of peripheral circadian clock by prostaglandin E2
Abstract
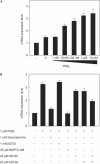
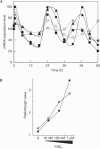
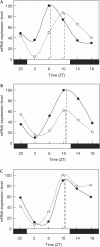
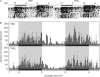
In mammals, the master circadian pacemaker is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN is thought to drive peripheral oscillators by controlling neuronal and humoral signals that can entrain the peripheral clocks. Here, we show that prostaglandin E2 (PGE2), a proinflammatory compound known to have diverse biological effects, is able to act as an in vivo clock-resetting agent. We find that in cultured NIH3T3 fibroblasts, PGE2 is able to induce transient expression of Period 1 messenger RNA and the following circadian oscillation of clock gene expression. Furthermore, we demonstrate that intraperitoneal administration of PGE2 results in the phase shift of circadian gene expression in mouse peripheral tissues in a time-dependent manner. This phase shift is also induced by the EP1/EP3 agonist sulprostone but not by the EP2 agonist butaprost. The PGE2-induced phase shift is inhibited by the EP1 antagonist SC-51322. These results suggest that PGE2 acts as an in vivo clock-resetting factor by means of the EP1 subtype of PGE receptors.
Figures

References
-
- Balsalobre A, Damiola F, Schibler U (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929–937 - PubMed
-
- Balsalobre A, Marcacci L, Schibler U (2000a) Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol 10: 1291–1294 - PubMed
-
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U (2000b) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347 - PubMed
-
- Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH (2004) Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol 36: 1187–1205 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

