Phytosphingosine induced mitochondria-involved apoptosis
- PMID: 15723652
- PMCID: PMC11159460
- DOI: 10.1111/j.1349-7006.2005.00012.x
Phytosphingosine induced mitochondria-involved apoptosis
Abstract
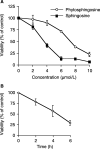
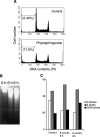
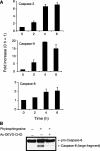
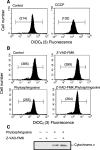
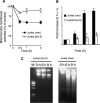
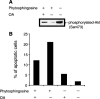
Sphingolipids are putative intracellular signal mediators in cell differentiation, growth inhibition, and apoptosis. Sphingosine, sphinganine, and phytosphingosine are structural analogs of sphingolipids and are classified as long-chain sphingoid bases. Sphingosine and sphinganine are known to play important roles in apoptosis. In the present study, we examined the phytosphingosine-induced apoptosis mechanism, focusing on mitochondria in human T-cell lymphoma Jurkat cells. Phytosphingosine significantly induced chromatin DNA fragmentation, which is a hallmark of apoptosis. Enzymatic activity measurements of caspases revealed that caspase-3 and caspase-9 are activated in phytosphingosine-induced apoptosis, but there is little activation of caspase-8 suggesting that phytosphingosine influences mitochondrial functions. In agreement with this hypothesis, a decrease in DeltaPsi(m) and the release of cytochrome c to the cytosol were observed upon phytosphingosine treatment. Furthermore, overexpression of mitochondria-localized anti-apoptotic protein Bcl-2 prevented phytosphingosine apoptotic stimuli. Western blot assays revealed that phytosphingosine decreases phosphorylated Akt and p70S6k. Dephosphorylation of Akt was partially inhibited by protein phosphatase inhibitor OA and OA attenuated phytosphingosine-induced apoptosis. Moreover, using a cell-free system, phytosphingosine directly reduced DeltaPsi(m). These results indicate that phytosphingosine perturbs mitochondria both directly and indirectly to induce apoptosis.
Figures


References
-
- Zamzami N, Marzo I, Susin SA, Brenner C, Larochette N, Marchetti P, Reed J, Kofler R, Kroemer G. The thiol crosslinking agent diamide overcomes the apoptosis‐inhibitory effect of Bcl‐2 by enforcing mitochondrial permeability transition. Oncogene 1998; 16: 1055–63. - PubMed
-
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell‐free extracts: requirement for dATP and cytochrome c . Cell 1996; 86: 147–57. - PubMed
-
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis‐inducing factor. Nature 1999; 397: 441–6. - PubMed
-
- Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 2000; 102: 43–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

