Selective inhibition of cyclooxygenase-2 inhibits colon cancer cell adhesion to extracellular matrix by decreased expression of beta1 integrin
- PMID: 15723653
- PMCID: PMC11159903
- DOI: 10.1111/j.1349-7006.2005.00022.x
Selective inhibition of cyclooxygenase-2 inhibits colon cancer cell adhesion to extracellular matrix by decreased expression of beta1 integrin
Abstract
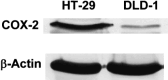
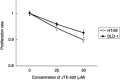
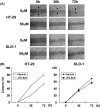
High level expression of cyclooxygenase (COX)-2 is reported in 80-90% of colorectal adenocarcinomas. In the recent years, selective inhibitors of COX-2 have been developed, and are shown to effectively protect against cancer development and progression. Colon cancer cells, as well as the epithelial cells in general, are dependent on appropriate interactions with the extracellular matrix (ECM) proteins to achieve a number of important functions, such as proliferation, differentiation, invasion and survival. These interactions are mediated via a family of cell-surface receptors called integrins, which interact with cytoskeletal proteins on the cytoplasmic side of the plasma membrane and thereby provide a link between the ECM and the cytoskeleton. In the present study, a high-COX-2 (high level COX-2 expression) colon cancer cell line, HT-29, and a low-COX-2 (low level COX-2 expression), DLD-1, were used to investigate the anticolon cancer effect of the selective COX-2 inhibitor, JTE-522. Moreover, to clarify its mechanisms of action, we focused especially on the ability to adhere to and to migrate on ECM. We could clearly demonstrate that, in addition to the decrease of the proliferative activity, JTE-522 caused a dose-dependent decrease in both the ability of colon cancer cells to adhere to and to migrate on ECM. These effects were, at least in part, dependent on the down-regulation of beta1-integrin expression, which was evident in HT-29, the high-COX-2 colon cancer cells, but not the low-COX-2, DLD-1. In addition, prostaglandin E2 almost completely reversed the effect of JTE-522, strongly suggesting the involvement of a COX-2-dependent pathway. In conclusion, for the first time, we could demonstrate the down-regulation of beta1 integrin caused by COX-2 inhibition, with consequent impairment of the ability of cancer cells to adhere to and to migrate on ECM, which are crucial steps for cancer metastases to develop.
Figures






Similar articles
-
The potential for a selective cyclooxygenase-2 inhibitor in the prevention of liver metastasis in human colorectal cancer.Anticancer Res. 2003 Jan-Feb;23(1A):245-9. Anticancer Res. 2003. PMID: 12680220
-
Suppression of pancreatic cancer cell invasion by a cyclooxygenase-2-specific inhibitor.Clin Exp Metastasis. 2003;20(7):577-84. doi: 10.1023/a:1027319903359. Clin Exp Metastasis. 2003. PMID: 14669788
-
Inhibition of haematogenous metastasis of colon cancer in mice by a selective COX-2 inhibitor, JTE-522.Br J Cancer. 1999 Dec;81(8):1274-9. doi: 10.1038/sj.bjc.6694262. Br J Cancer. 1999. PMID: 10604722 Free PMC article.
-
[COX-2 inhibitor and colon cancer].Gan To Kagaku Ryoho. 2001 Nov;28(12):1799-805. Gan To Kagaku Ryoho. 2001. PMID: 11729471 Review. Japanese.
-
Analysis of Nanoparticle Effects on Invasion of Cancer Cells: Version 1.0.2020 May. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method IEA-1. 2020 May. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method IEA-1. PMID: 39012996 Free Books & Documents. Review.
Cited by
-
COX-2 expression in gastric cancer and its relationship with angiogenesis using tissue microarray.World J Gastroenterol. 2007 Jul 7;13(25):3466-71. doi: 10.3748/wjg.v13.i25.3466. World J Gastroenterol. 2007. PMID: 17659693 Free PMC article.
-
Prostaglandins in cancer cell adhesion, migration, and invasion.Int J Cell Biol. 2012;2012:723419. doi: 10.1155/2012/723419. Epub 2012 Feb 29. Int J Cell Biol. 2012. PMID: 22505934 Free PMC article.
-
The tumor microenvironment in colorectal carcinogenesis.Cancer Microenviron. 2010 Mar 5;3(1):149-66. doi: 10.1007/s12307-010-0038-3. Cancer Microenviron. 2010. PMID: 21209781 Free PMC article.
-
Prostaglandin E2 stimulates β1-integrin expression in hepatocellular carcinoma through the EP1 receptor/PKC/NF-κB pathway.Sci Rep. 2014 Oct 7;4:6538. doi: 10.1038/srep06538. Sci Rep. 2014. PMID: 25289898 Free PMC article.
-
Cyclo-oxygenase-2 and its inhibition in cancer: is there a role?Drugs. 2007;67(6):821-45. doi: 10.2165/00003495-200767060-00001. Drugs. 2007. PMID: 17428102 Review.
References
-
- Church RD, Fleshman JW, McLeod HL. Cyclo‐oxygenase 2 inhibition in colorectal cancer therapy. Br J Surg 2003; 90: 1055–67. - PubMed
-
- Gasparini G, Longo R, Sarmiento R, Morabito A. Inhibitors of cyclo‐oxygenase 2: a new class of anticancer agents? Lancet Oncol 2003; 4: 605–15. - PubMed
-
- Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer 1997; 80: 1529–37. - PubMed
-
- Varner JA. Cheresh DA. Integrins and cancer. Curr Opin Cell Biol 1996; 8: 724–30. - PubMed
-
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992; 69: 11–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

