A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer
- PMID: 15723705
- PMCID: PMC551593
- DOI: 10.1186/1479-5876-3-9
A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer
Abstract
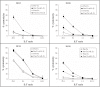
BACKGROUND: There is a continued need to develop more effective cancer immunotherapy strategies. Exosomes, cell-derived lipid vesicles that express high levels of a narrow spectrum of cell proteins represent a novel platform for delivering high levels of antigen in conjunction with costimulatory molecules. We performed this study to test the safety, feasibility and efficacy of autologous dendritic cell (DC)-derived exosomes (DEX) loaded with the MAGE tumor antigens in patients with non-small cell lung cancer (NSCLC). METHODS: This Phase I study enrolled HLA A2+ patients with pre-treated Stage IIIb (N = 4) and IV (N = 9) NSCLC with tumor expression of MAGE-A3 or A4. Patients underwent leukapheresis to generate DC from which DEX were produced and loaded with MAGE-A3, -A4, -A10, and MAGE-3DPO4 peptides. Patients received 4 doses of DEX at weekly intervals. RESULTS: Thirteen patients were enrolled and 9 completed therapy. Three formulations of DEX were evaluated; all were well tolerated with only grade 1-2 adverse events related to the use of DEX (injection site reactions (N = 8), flu like illness (N = 1), and peripheral arm pain (N = 1)). The time from the first dose of DEX until disease progression was 30 to 429+ days. Three patients had disease progression before the first DEX dose. Survival of patients after the first DEX dose was 52-665+ days. DTH reactivity against MAGE peptides was detected in 3/9 patients. Immune responses were detected in patients as follows: MAGE-specific T cell responses in 1/3, increased NK lytic activity in 2/4. CONCLUSION: Production of the DEX vaccine was feasible and DEX therapy was well tolerated in patients with advanced NSCLC. Some patients experienced long term stability of disease and activation of immune effectors.
Figures
References
-
- Shichijo S, Hayashi A, Takamori S, Tsunosue R, Hoshino T, Sakata M, Kuramoto T, Oizumi K, Itoh K. Detection of MAGE-4 protein in lung cancers. Int J Cancer. 1995;64:158–65. - PubMed
-
- Reynolds SR, Zeleniuch-Jacquotte A, Shapiro RL, Roses DF, Harris MN, Johnston D, Bystryn JC. Vaccine-induced CD8+ T-cell responses to MAGE-3 correlate with clinical outcome in patients with melanoma. Clin Cancer Res. 2003;9:657–62. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

