Identification and functional characterization of a novel Candida albicans gene CaMNN5 that suppresses the iron-dependent growth defect of Saccharomyces cerevisiae aft1Delta mutant
- PMID: 15725072
- PMCID: PMC1184536
- DOI: 10.1042/BJ20050223
Identification and functional characterization of a novel Candida albicans gene CaMNN5 that suppresses the iron-dependent growth defect of Saccharomyces cerevisiae aft1Delta mutant
Abstract
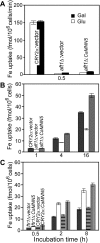
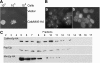
In Saccharomyces cerevisiae, the transcription factor Aft1p plays a central role in regulating many genes involved in iron acquisition and utilization. An aft1Delta mutant exhibits severely retarded growth under iron starvation. To identify the functional counterpart of AFT1 in Candida albicans, we transformed a C. albicans genomic DNA library into aft1Delta to isolate genes that could allow the mutant to grow under iron-limiting conditions. In the present paper, we describe the unexpected discovery in this screen of CaMNN5. CaMnn5p is an alpha-1,2-mannosyltransferease, but its growth-promoting function in iron-limiting conditions does not require this enzymatic activity. Its function is also independent of the high-affinity iron transport systems that are mediated by Ftr1p and Fth1p. We obtained evidence suggesting that CaMnn5p may function along the endocytic pathway, because it cannot promote the growth of end4Delta and vps4Delta mutants, where the endocytic pathway is blocked at an early and late step respectively. Neither can it promote the growth of a fth1Delta smf3Delta mutant, where the vacuole-cytosol iron transport is blocked. Expression of CaMNN5 in S. cerevisiae specifically enhances an endocytosis-dependent mechanism of iron uptake without increasing the uptake of Lucifer Yellow, a marker for fluid-phase endocytosis. CaMnn5p contains three putative Lys-Glu-Xaa-Xaa-Glu iron-binding sites and co-immunoprecipitates with 55Fe. We propose that CaMnn5p promotes iron uptake and usage along the endocytosis pathway under iron-limiting conditions, a novel function that might have evolved in C. albicans.
Figures





Similar articles
-
Isolation of a Candida albicans gene, tightly linked to URA3, coding for a putative transcription factor that suppresses a Saccharomyces cerevisiae aft1 mutation.Yeast. 2001 Mar 15;18(4):301-11. doi: 10.1002/1097-0061(20010315)18:4<301::AID-YEA672>3.0.CO;2-H. Yeast. 2001. PMID: 11223939
-
[CaSRB9, a novel Candida albicans gene, plays a role in morphogenesis of Saccharomyces cerevisiae].Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 2002 May;34(3):298-304. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 2002. PMID: 12019441 Chinese.
-
An endocytic mechanism for haemoglobin-iron acquisition in Candida albicans.Mol Microbiol. 2008 Jul;69(1):201-17. doi: 10.1111/j.1365-2958.2008.06277.x. Epub 2008 May 5. Mol Microbiol. 2008. PMID: 18466294
-
Coregulation of starch degradation and dimorphism in the yeast Saccharomyces cerevisiae.Crit Rev Biochem Mol Biol. 1997;32(5):405-35. doi: 10.3109/10409239709082675. Crit Rev Biochem Mol Biol. 1997. PMID: 9383611 Review.
-
Co-regulation of pathogenesis with dimorphism and phenotypic switching in Candida albicans, a commensal and a pathogen.Int J Med Microbiol. 2002 Oct;292(5-6):299-311. doi: 10.1078/1438-4221-00215. Int J Med Microbiol. 2002. PMID: 12452278 Review.
Cited by
-
Robustness analysis on interspecies interaction network for iron and glucose competition between Candida albicans and zebrafish during infection.BMC Syst Biol. 2014;8 Suppl 5(Suppl 5):S6. doi: 10.1186/1752-0509-8-S5-S6. Epub 2014 Dec 12. BMC Syst Biol. 2014. PMID: 25603810 Free PMC article.
-
Iron binding modulates candidacidal properties of salivary histatin 5.J Dent Res. 2015 Jan;94(1):201-8. doi: 10.1177/0022034514556709. Epub 2014 Nov 3. J Dent Res. 2015. PMID: 25365968 Free PMC article.
-
MNN5 encodes an iron-regulated alpha-1,2-mannosyltransferase important for protein glycosylation, cell wall integrity, morphogenesis, and virulence in Candida albicans.Eukaryot Cell. 2006 Feb;5(2):238-47. doi: 10.1128/EC.5.2.238-247.2006. Eukaryot Cell. 2006. PMID: 16467465 Free PMC article.
References
-
- Hill H. A. O. Iron: an element well-fitted for its task? In: Dunford H. B., Dolphin D., Raymond K. N., Seiker L., editors. The Biological Chemistry of Iron. Reidel, Dordrecht; 1982. pp. 1–12.
-
- Crichton R. R., Charloteaux-Wauters M. Iron transport and storage. Eur. J. Biochem. 1987;164:485–506. - PubMed
-
- Elin R. J., Wolff S. M. Effect of pH and iron concentration on growth of Candida albicans in human serum. J. Infect. Dis. 1973;127:705–708. - PubMed
-
- Ramanan N., Wang Y. A high-affinity iron permease essential for Candida albicans virulence. Science. 2000;288:1062–1064. - PubMed
-
- Griffiths E. Iron in biological systems. In: Bullen J. J., Griffiths E., editors. Iron and Infection: Molecular, Clinical, and Physiological Aspects. Chichester: Wiley; 1999. pp. 1–25.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

