Cisplatin plus oral etoposide (EoP) combination is more effective than paclitaxel in patients with advanced breast cancer pretreated with anthracyclines: a randomised phase III trial of Turkish Oncology Group
- PMID: 15726120
- PMCID: PMC2361864
- DOI: 10.1038/sj.bjc.6602388
Cisplatin plus oral etoposide (EoP) combination is more effective than paclitaxel in patients with advanced breast cancer pretreated with anthracyclines: a randomised phase III trial of Turkish Oncology Group
Abstract
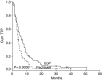
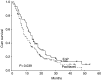
Our objective was to determine whether oral etoposide and cisplatin combination (EoP) is superior to paclitaxel in the treatment of advanced breast cancer (ABC) patients pretreated with anthracyclines. From December 1997 to August 2003, 201 patients were randomised, 100 to EoP and 101 to paclitaxel arms. Four patients in each arm were ineligible. The doses of etoposide and cisplatin were 50 mg p.o. twice a day for 7 days and 70 mg m(-2) intravenously (i.v.) on day 1, respectively, and it was 175 mg m(-2) on day 1 for paclitaxel. Both treatments were repeated every 3 weeks. A median of four cycles of study treatment was given in both arms. The response rate obtained in the EoP arm was significantly higher (36.3 vs 22.2%; P=0.038). Median response duration was longer for the EoP arm (7 vs 4 months) (P=0.132). Also, time to progression was significantly in favour of the EoP arm (5.5 vs 3.9 months; P=0.003). Median overall survival was again significantly longer in the EoP arm (14 vs 9.5 months; P=0.039). Toxicity profile of both groups was similar. Two patients in each arm were lost due to febrile neutropenia. The observed activity and acceptable toxicity of EoP endorses the employment of this combination in the treatment of ABC following anthracyclines.
Figures
References
-
- Abrams JS, Vena DA, Baltz J, Adams J, Montello M, Christian M, Onetto N, Desmond-Hellmann S, Canetta R, Friedman MA (1995) Paclitaxel activity in heavily pretreated breast cancer: a National Cancer Institute referral center. J Clin Oncol 13: 2056–2065 - PubMed
-
- Athanassiades P, Bacoyiannes H, Kontoyiannes D (1986) Etoposide and cisdichlorodiammine platinum in advanced breast carcinoma resistant to previous chemotherapy. Chemioterapia 5: 125–127 - PubMed
-
- Atienza DM, Vogel CL, Trock B, Swain SM (1995) Phase II study of oral etoposide for patients with advanced breast cancer. Cancer 12: 2485–2490 - PubMed
-
- Bontenbal M, Planting AS, Verweij J, de Wit R, Kruit WH, Stoter G, Klijn JG (1995) Second-line chemotherapy with long-term low-dose oral etoposide in patients with advanced breast cancer. Breast Cancer Res Treat 34: 185–189 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical