Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury
- PMID: 15726663
- PMCID: PMC1821079
- DOI: 10.1002/hep.20600
Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury
Abstract
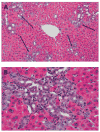
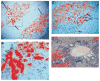
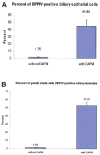
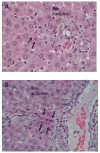
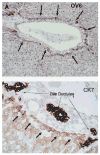
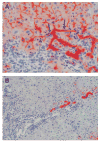
Rats with chimeric livers were generated by using the protocol of injecting hepatocytes from dipeptidyl peptidase IV (DPPIV)-positive donors into retrorsine-treated DPPIV-negative recipients subjected to partial hepatectomy. Rats with established chimeric livers were subjected to bile duct ligation, with or without pretreatment with the biliary toxin methylene diamiline (DAPM). Ductules bearing the donor hepatocyte marker DPPIV were seen at 30 days after bile duct ligation. The frequency of the ductules derived from the donor hepatocytes was dramatically enhanced (36-fold) by the pretreatment with DAPM. In conclusion, our results show that hepatocytes can function as facultative stem cells and rescue the biliary epithelium during repair from injury when its proliferative capacity is being compromised.
Conflict of interest statement
Conflict of interest: Nothing to report.
Figures



References
-
- Alison M, Golding M, Lalani EN, Nagy P, Thorgeirsson S, Sarraf C. Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J Hepatol. 1997;26:343–352. - PubMed
-
- Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737–1740. - PubMed
-
- Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–1547. - PubMed
-
- Evarts RP, Hu Z, Omori N, Omori M, Marsden ER, Thorgeirsson SS. Precursor-product relationship between oval cells and hepatocytes: comparison between tritiated thymidine and bromodeoxyuridine as tracers. Carcinogenesis. 1996;17:2143–2151. - PubMed
-
- Golding M, Sarraf CE, Lalani EN, Anilkumar TV, Edwards RJ, Nagy P, et al. Oval cell differentiation into hepatocytes in the acetylaminofluorene-treated regenerating rat liver. Hepatology. 1995;22:1243–1253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
