Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence
- PMID: 15727532
- PMCID: PMC2430889
- DOI: 10.1037/0735-7044.119.1.280
Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence
Abstract
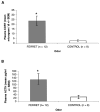
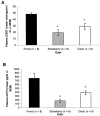
Predator odors have been shown to elicit stress responses in rats. The present studies assessed the use of domestic ferret odor as a processive stress model. Plasma corticosterone and adrenocorticotropin hormone levels were higher after 30 min of exposure to ferret odor (fur/skin) but not control odors, ferret feces, urine, or anal gland secretions. Behavioral differences were also found between ferret and the control odors as tested in a defensive withdrawal paradigm. In addition, c-fos messenger RNA expression in several brain areas previously associated with processive stress was significantly higher in ferret odor-exposed rat brains than in control odor-exposed brains. These results suggest that ferret odor produces a reliable unconditioned stress response and may be useful as a processive stress model.
Copyright 2005 APA.
Figures



References
-
- Anisman H, Lu ZW, Song C, Kent P, McIntyre DC, Merali Z. Influence of psychogenic and neurogenic stressors on endocrine and immune activity: Differential effects in fast and slow seizing rat strains. Brain, Behavior, and Immunity. 1997;11:63–74. - PubMed
-
- Apfelbach R. Instinctive predatory behavior of the ferret (Putorius putorius furo L.) modified by chlordiazepoxide hydrochloride (Librium) Psychopharmacology. 1978;59:179–182. - PubMed
-
- Blanchard DC, Blanchard RJ. Ethobiological approaches to the biology of emotion. Annual Review of Psychology. 1988;39:43–68. - PubMed
-
- Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: Some reflections on stress and emotion. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:1177–1185. - PubMed

